The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin
- PMID: 19246757
- PMCID: PMC10010501
- DOI: 10.1099/mic.0.024604-0
The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin
Abstract
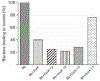
The Lyme disease spirochaete, Borrelia burgdorferi, can invade and persistently infect its hosts' connective tissues. We now demonstrate that B. burgdorferi adheres to the extracellular matrix component laminin. The surface-exposed outer-membrane protein ErpX was identified as having affinity for laminin, and is the first laminin-binding protein to be identified in a Lyme disease spirochaete. The adhesive domain of ErpX was shown to be contained within a small, unstructured hydrophilic segment at the protein's centre. The sequence of that domain is distinct from any previously identified bacterial laminin adhesin, suggesting a unique mode of laminin binding.
Figures


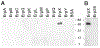


Similar articles
-
Borrelia burgdorferi BmpA is a laminin-binding protein.Infect Immun. 2009 Nov;77(11):4940-6. doi: 10.1128/IAI.01420-08. Epub 2009 Aug 24. Infect Immun. 2009. PMID: 19703983 Free PMC article.
-
Cross-species surface display of functional spirochetal lipoproteins by recombinant Borrelia burgdorferi.Infect Immun. 2004 Mar;72(3):1463-9. doi: 10.1128/IAI.72.3.1463-1469.2004. Infect Immun. 2004. PMID: 14977951 Free PMC article.
-
Borrelia burgdorferi RevA antigen binds host fibronectin.Infect Immun. 2009 Jul;77(7):2802-12. doi: 10.1128/IAI.00227-09. Epub 2009 Apr 27. Infect Immun. 2009. PMID: 19398540 Free PMC article.
-
Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection.Int J Med Microbiol. 2008 Sep 1;298 Suppl 1(Suppl 1):257-67. doi: 10.1016/j.ijmm.2007.09.004. Epub 2008 Jan 11. Int J Med Microbiol. 2008. PMID: 18248770 Free PMC article. Review.
-
Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi.Biochem Soc Trans. 2003 Feb;31(Pt 1):108-12. doi: 10.1042/bst0310108. Biochem Soc Trans. 2003. PMID: 12546665 Review.
Cited by
-
Identification and functional characterisation of Complement Regulator Acquiring Surface Protein-1 of serum resistant Borrelia garinii OspA serotype 4.BMC Microbiol. 2010 Feb 10;10:43. doi: 10.1186/1471-2180-10-43. BMC Microbiol. 2010. PMID: 20146822 Free PMC article.
-
Borrelia burgdorferi cp32 BpaB modulates expression of the prophage NucP nuclease and SsbP single-stranded DNA-binding protein.J Bacteriol. 2012 Sep;194(17):4570-8. doi: 10.1128/JB.00661-12. Epub 2012 Jun 22. J Bacteriol. 2012. PMID: 22730122 Free PMC article.
-
Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins.J Bacteriol. 2013 Feb;195(4):757-64. doi: 10.1128/JB.01956-12. Epub 2012 Dec 7. J Bacteriol. 2013. PMID: 23222718 Free PMC article.
-
Multifunctional and Redundant Roles of Borrelia burgdorferi Outer Surface Proteins in Tissue Adhesion, Colonization, and Complement Evasion.Front Immunol. 2016 Oct 21;7:442. doi: 10.3389/fimmu.2016.00442. eCollection 2016. Front Immunol. 2016. PMID: 27818662 Free PMC article. Review.
-
Erp and Rev Adhesins of the Lyme Disease Spirochete's Ubiquitous cp32 Prophages Assist the Bacterium during Vertebrate Infection.Infect Immun. 2023 Mar 15;91(3):e0025022. doi: 10.1128/iai.00250-22. Epub 2023 Feb 28. Infect Immun. 2023. PMID: 36853019 Free PMC article. Review.
References
-
- Alitalo A, Meri T, Lankinen H, Seppälä I, Lahdenne P, Hefty PS, Akins D & Meri S (2002). Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J Immunol 169, 3847–3853. - PubMed
-
- Balmelli T & Piffaretti JC (1995). Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol 146, 329–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

