Evaluation of procedures for outer membrane isolation from Campylobacter jejuni
- PMID: 19246768
- PMCID: PMC2763183
- DOI: 10.1099/mic.0.024539-0
Evaluation of procedures for outer membrane isolation from Campylobacter jejuni
Abstract
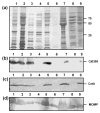
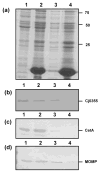
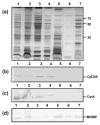
Although infection with Campylobacter jejuni is one of the leading causes of gastroenteritis worldwide, relatively little is known about the factors that are required to elicit a protective immune response. The need for a vaccine against this pathogen is well recognized and a number of vaccine candidates have been tested with varying degrees of success; however, there is still a lack of a suitable vaccine. To gain a better understanding of the outer-membrane protein components of this organism, a 'gold standard' method to purify the outer membrane is needed. Therefore, we attempted to develop a robust and reliable method which resulted in a pure outer-membrane fraction. A total of nine methodologies were examined and analysed by SDS-PAGE and immunoblotting using subcellular markers for the cytoplasm, cytoplasmic membrane and outer membrane. We found that glycine extraction, differential detergent extraction using Triton X-100, serial extraction using 1 M Tris pH 7, spheroplasting by lysozyme and sonication, and carbonate extraction did not produce pure outer-membrane preparations. However, we identified three methods that provided outer-membrane fractions free from subcellular contamination. Isopycnic centrifugation using a 30-60 % sucrose gradient produced seven fractions free from cytoplasmic or cytoplasmic membrane contamination; however, these fractions did not correspond as well as expected with the typical outer-membrane-associated peak (e.g. Escherichia coli or Salmonella). The spheroplast method using lysozyme alone also resulted in pure outer-membrane fraction, as did carbonate washing of this sample. The extraction of outer membranes using N-lauroylsarcosine (Sarkosyl) produced the purest and most reproducible sample. These outer-membrane preparations will be useful for future studies aimed at identifying C. jejuni surface proteins as vaccine components.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

