Gene dosage effects of the imprinted delta-like homologue 1 (dlk1/pref1) in development: implications for the evolution of imprinting
- PMID: 19247431
- PMCID: PMC2640098
- DOI: 10.1371/journal.pgen.1000392
Gene dosage effects of the imprinted delta-like homologue 1 (dlk1/pref1) in development: implications for the evolution of imprinting
Abstract
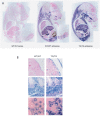
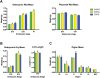
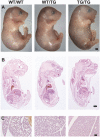
Genomic imprinting is a normal process that causes genes to be expressed according to parental origin. The selective advantage conferred by imprinting is not understood but is hypothesised to act on dosage-critical genes. Here, we report a unique model in which the consequences of a single, double, and triple dosage of the imprinted Dlk1/Pref1, normally repressed on the maternally inherited chromosome, can be assessed in the growing embryo. BAC-transgenic mice were generated that over-express Dlk1 from endogenous regulators at all sites of embryonic activity. Triple dosage causes lethality associated with major organ abnormalities. Embryos expressing a double dose of Dlk1, recapitulating loss of imprinting, are growth enhanced but fail to thrive in early life, despite the early growth advantage. Thus, any benefit conferred by increased embryonic size is offset by postnatal lethality. We propose a negative correlation between gene dosage and survival that fixes an upper limit on growth promotion by Dlk1, and we hypothesize that trade-off between growth and lethality might have driven imprinting at this locus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

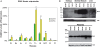

References
-
- da Rocha ST, Ferguson-Smith AC. Genomic imprinting. Curr Biol. 2004;14:R646–649. - PubMed
-
- Takada S, Tevendale M, Baker J, Georgiades P, Campbell E, et al. Delta-like and Gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol. 2000;10:1135–1138. - PubMed
-
- Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. - PubMed
-
- Baladron V, Ruiz-Hidalgo MJ, Nueda ML, Diaz-Guerra MJ, Garcia-Ramirez JJ, et al. Dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res. 2005;303:343–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

