Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion
- PMID: 19247437
- PMCID: PMC2642630
- DOI: 10.1371/journal.ppat.1000309
Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion
Abstract
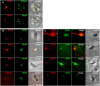
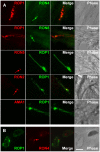
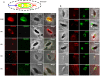
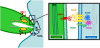
One of the most conserved features of the invasion process in Apicomplexa parasites is the formation of a moving junction (MJ) between the apex of the parasite and the host cell membrane that moves along the parasite and serves as support to propel it inside the host cell. The MJ was, up to a recent period, completely unknown at the molecular level. Recently, proteins originated from two distinct post-Golgi specialised secretory organelles, the micronemes (for AMA1) and the neck of the rhoptries (for RON2/RON4/RON5 proteins), have been shown to form a complex. AMA1 and RON4 in particular, have been localised to the MJ during invasion. Using biochemical approaches, we have identified RON8 as an additional member of the complex. We also demonstrated that all RON proteins are present at the MJ during invasion. Using metabolic labelling and immunoprecipitation, we showed that RON2 and AMA1 were able to interact in the absence of the other members. We also discovered that all MJ proteins are subjected to proteolytic maturation during trafficking to their respective organelles and that they could associate as non-mature forms in vitro. Finally, whereas AMA1 has previously been shown to be inserted into the parasite membrane upon secretion, we demonstrated, using differential permeabilization and loading of RON-specific antibodies into the host cell, that the RON complex is targeted to the host cell membrane, where RON4/5/8 remain associated with the cytoplasmic face. Globally, these results point toward a model of MJ organization where the parasite would be secreting and inserting interacting components on either side of the MJ, both at the host and at its own plasma membranes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
-
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. - PubMed
-
- Dubremetz JF. Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction. Cell Microbiol. 2007;9:841–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

