Simultaneous quantification of buprenorphine, norbuprenorphine, buprenorphine glucuronide, and norbuprenorphine glucuronide in human placenta by liquid chromatography mass spectrometry
- PMID: 19247639
- PMCID: PMC3159561
- DOI: 10.1007/s00216-009-2706-z
Simultaneous quantification of buprenorphine, norbuprenorphine, buprenorphine glucuronide, and norbuprenorphine glucuronide in human placenta by liquid chromatography mass spectrometry
Abstract
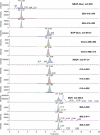
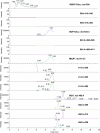
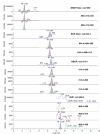
A LCMS method was developed and validated for the determination of buprenorphine (BUP), norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc), and norbuprenorphine glucuronide (NBUP-Gluc) in placenta. Quantification was achieved by selected ion monitoring of m/z 468.4 (BUP), 414.3 (NBUP), 644.4 (BUP-Gluc), and 590 (NBUP-Gluc). BUP and NBUP were identified monitoring MS(2) fragments m/z 396, 414 and 426 for BUP, and 340, 364 and 382 for NBUP, and glucuronide conjugates monitoring MS(3) fragments m/z 396 and 414 for BUP-Gluc, and 340 and 382 for NBUP-Gluc. Linearity was 1-50 ng/g. Intra-day, inter-day and total assay imprecision (% RSD) were <13.4%, and analytical recoveries were 96.2-113.1%. Extraction efficiencies ranged from 40.7-68%, process efficiencies 38.8-70.5%, and matrix effect 1.3-15.4%. Limits of detection were 0.8 ng/g for all compounds. An authentic placenta from an opioid-dependent pregnant woman receiving BUP pharmacotherapy was analyzed. BUP was not detected but metabolite concentrations were NBUP-Gluc 46.6, NBUP 15.7 and BUP-Gluc 3.2 ng/g.
Figures
Similar articles
-
Confirmatory analysis of buprenorphine, norbuprenorphine, and glucuronide metabolites in plasma by LCMSMS. Application to umbilical cord plasma from buprenorphine-maintained pregnant women.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jan 1;878(1):13-20. doi: 10.1016/j.jchromb.2009.11.005. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 19945361 Free PMC article.
-
Simultaneous quantification of buprenorphine, norbuprenorphine, buprenorphine-glucuronide and norbuprenorphine-glucuronide in human umbilical cord by liquid chromatography tandem mass spectrometry.Forensic Sci Int. 2009 Jul 1;188(1-3):144-51. doi: 10.1016/j.forsciint.2009.04.005. Epub 2009 Apr 29. Forensic Sci Int. 2009. PMID: 19406593 Free PMC article.
-
Urinary excretion of buprenorphine, norbuprenorphine, buprenorphine-glucuronide, and norbuprenorphine-glucuronide in pregnant women receiving buprenorphine maintenance treatment.Clin Chem. 2009 Jun;55(6):1177-87. doi: 10.1373/clinchem.2008.113712. Epub 2009 Mar 26. Clin Chem. 2009. PMID: 19325013 Free PMC article. Clinical Trial.
-
Development and validation of a liquid chromatography-tandem mass spectrometry assay for the simultaneous quantification of buprenorphine, norbuprenorphine, and metabolites in human urine.Anal Bioanal Chem. 2008 Nov;392(5):903-11. doi: 10.1007/s00216-008-2326-z. Epub 2008 Aug 30. Anal Bioanal Chem. 2008. PMID: 18758763 Free PMC article.
-
Determination of buprenorphine, norbuprenorphine, naloxone, and their glucuronides in urine by liquid chromatography-tandem mass spectrometry.Drug Test Anal. 2021 Sep;13(9):1658-1667. doi: 10.1002/dta.3104. Epub 2021 Jun 3. Drug Test Anal. 2021. PMID: 34047070
Cited by
-
Predictors of neonatal abstinence syndrome in buprenorphine exposed newborn: can cord blood buprenorphine metabolite levels help?Springerplus. 2016 Jun 23;5(1):854. doi: 10.1186/s40064-016-2576-8. eCollection 2016. Springerplus. 2016. PMID: 27386303 Free PMC article.
-
Maternal methadone dose, placental methadone concentrations, and neonatal outcomes.Clin Chem. 2011 Mar;57(3):449-58. doi: 10.1373/clinchem.2010.154864. Epub 2011 Jan 18. Clin Chem. 2011. PMID: 21245372 Free PMC article. Clinical Trial.
-
A Pharmacologic Evaluation of Buprenorphine in Pregnancy and the Postpartum Period.J Addict Med. 2025 Mar-Apr 01;19(2):129-134. doi: 10.1097/ADM.0000000000001380. Epub 2024 Sep 2. J Addict Med. 2025. PMID: 39221812
-
Confirmatory analysis of buprenorphine, norbuprenorphine, and glucuronide metabolites in plasma by LCMSMS. Application to umbilical cord plasma from buprenorphine-maintained pregnant women.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jan 1;878(1):13-20. doi: 10.1016/j.jchromb.2009.11.005. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 19945361 Free PMC article.
References
-
- Drug Addiction Treatment Act of 2000. 2000. p. 111. STAT. 1101.
-
- Lejeune C, Simmat-Durand L, Gourarier L, et al. Drug Alcohol Depend. 2006;82:250–257. - PubMed
-
- Fischer G, Johnson RE, Eder H, et al. Addiction. 2000;95(2):239–244. - PubMed
-
- Dunlop AJ, Panjari M, O'Sullivan H, et al. Clinical guidelines for the use of buprenorphine in pregnancy. Turning Point Alcohol and Drug Centre; Fitzroy: 2003.
-
- Jones HE, Johnson RE, Jasinski DR, et al. Drug Alcohol Depend. 2005;79:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

