Attachment of osteocyte cell processes to the bone matrix
- PMID: 19248169
- PMCID: PMC2861341
- DOI: 10.1002/ar.20869
Attachment of osteocyte cell processes to the bone matrix
Abstract
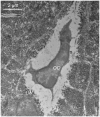
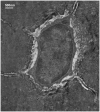
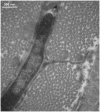
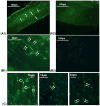
In order for osteocytes to perceive mechanical information and regulate bone remodeling accordingly they must be anchored to their extracellular matrix (ECM). To date the nature of this attachment is not understood. Osteocytes are embedded in mineralized bone matrix, but maintain a pericellular space (50-80 nm) to facilitate fluid flow and transport of metabolites. This provides a spatial limit for their attachment to bone matrix. Integrins are cell adhesion proteins that may play a role in osteocyte attachment. However, integrin attachments require proximity between the ECM, cell membrane, and cytoskeleton, which conflicts with the osteocytes requirement for a pericellular fluid space. In this study, we hypothesize that the challenge for osteocytes to attach to surrounding bone matrix, while also maintaining fluid-filled pericellular space, requires different "engineering" solutions than in other tissues that are not similarly constrained. Using novel rapid fixation techniques, to improve cell membrane and matrix protein preservation, and transmission electron microscopy, the attachment of osteocyte processes to their canalicular boundaries are quantified. We report that the canalicular wall is wave-like with periodic conical protrusions extending into the pericellular space. By immunohistochemistry we identify that the integrin alphavbeta3 may play a role in attachment at these complexes; a punctate pattern of staining of beta3 along the canalicular wall was consistent with observations of periodic protrusions extending into the pericellular space. We propose that during osteocyte attachment the pericellular space is periodically interrupted by underlying collagen fibrils that attach directly to the cell process membrane via integrin-attachments.
(c) 2009 Wiley-Liss, Inc.
Figures



References
-
- Aarden EM, Nijweide PJ, Van Der Plas A, Alblas MJ, Mackie EJ, Horton MA, Helfrich MH. Adhesive properties of isolated chick osteocytes in vitro. Bone. 1996;18:305–313. - PubMed
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. - PubMed
-
- Ayasaka N, Kondo T, Goto T, Kido MA, Nagata E, Tanaka T. Differences in the transport systems between cementocytes and osteocytes in rats using microperoxidase as a tracer. Arch Oral Biol. 1992;37:363–369. - PubMed
-
- Bell K, Loveridge N, Power J, Garrahan N, Meggitt B, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24(1):57–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

