Point-of-care continuous (13)C-methacetin breath test improves decision making in acute liver disease: results of a pilot clinical trial
- PMID: 19248196
- PMCID: PMC2653395
- DOI: 10.3748/wjg.15.966
Point-of-care continuous (13)C-methacetin breath test improves decision making in acute liver disease: results of a pilot clinical trial
Abstract
Aim: To assess the role of the (13)C-methacetin breath test (MBT) in patients with acute liver disease.
Methods: Fifteen patients with severe acute liver disease from diverse etiologies were followed-up with (13)C-MBT during the acute phase of their illnesses (range 3-116 d after treatment). Patients fasted for 8 h and ingested 75 mg of methacetin prior to the MBT. We compared results from standard clinical assessment, serum liver enzymes, synthetic function, and breath test scores.
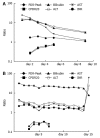
Results: Thirteen patients recovered and two patients died. In patients that recovered, MBT parameters improved in parallel with improvements in lab results. Evidence of consistent improvement began on day 3 for MBT parameters and between days 7 and 9 for blood tests. Later convergence to normality occurred at an average of 9 d for MBT parameters and from 13 to 28 d for blood tests. In both patients that died, MBT parameters remained low despite fluctuating laboratory values.
Conclusion: The (13)C-MBT provides a rapid, non-invasive assessment of liver function in acute severe liver disease of diverse etiologies. The results of this pilot clinical trial suggest that the MBT may offer greater sensitivity than standard clinical tests for managing patients with severe acute liver disease.
Figures
Similar articles
-
Prognostic Value of the 13 C-Methacetin Breath Test in Adults with Acute Liver Failure and Non-acetaminophen Acute Liver Injury.Hepatology. 2021 Aug;74(2):961-972. doi: 10.1002/hep.31783. Hepatology. 2021. PMID: 33660316 Free PMC article.
-
Interference of acute cigarette smoking with [¹³C]methacetin breath test.Isotopes Environ Health Stud. 2011 Mar;47(1):34-41. doi: 10.1080/10256016.2010.549229. Isotopes Environ Health Stud. 2011. PMID: 21287423
-
Gd-EOB-DTPA-enhanced T1 relaxometry for assessment of liver function determined by real-time 13C-methacetin breath test.Eur Radiol. 2018 Sep;28(9):3591-3600. doi: 10.1007/s00330-018-5337-y. Epub 2018 Mar 12. Eur Radiol. 2018. PMID: 29532241
-
Review article: the assessment of liver function using breath tests.Aliment Pharmacol Ther. 2007 Nov 15;26(10):1293-302. doi: 10.1111/j.1365-2036.2007.03519.x. Epub 2007 Sep 15. Aliment Pharmacol Ther. 2007. PMID: 17868431 Review.
-
Critical appraisal of 13C breath tests for microsomal liver function: aminopyrine revisited.Liver Int. 2014 Apr;34(4):487-94. doi: 10.1111/liv.12451. Epub 2014 Jan 16. Liver Int. 2014. PMID: 24428683 Review.
Cited by
-
C-methacetin breath test reproducibility study reveals persistent CYP1A2 stimulation on repeat examinations.World J Gastroenterol. 2011 Dec 7;17(45):4979-86. doi: 10.3748/wjg.v17.i45.4979. World J Gastroenterol. 2011. PMID: 22174547 Free PMC article.
-
Serum Levels of Adropin Improve the Predictability of MELD and Child-Pugh Score in Cirrhosis: Results of Proof-of-Concept Clinical Trial.Transpl Int. 2023 Jun 2;36:11176. doi: 10.3389/ti.2023.11176. eCollection 2023. Transpl Int. 2023. PMID: 37334012 Free PMC article.
-
Prognostic Value of the 13 C-Methacetin Breath Test in Adults with Acute Liver Failure and Non-acetaminophen Acute Liver Injury.Hepatology. 2021 Aug;74(2):961-972. doi: 10.1002/hep.31783. Hepatology. 2021. PMID: 33660316 Free PMC article.
-
A rapid non invasive L-DOPA-¹³C breath test for optimally suppressing extracerebral AADC enzyme activity - toward individualizing carbidopa therapy in Parkinson’s disease.J Parkinsons Dis. 2012;2(4):349-56. doi: 10.3233/JPD-012132. J Parkinsons Dis. 2012. PMID: 23646099 Free PMC article. Clinical Trial.
-
(13)CO2 breath tests in non-invasive hepatological diagnosis.Prz Gastroenterol. 2015;10(1):1-6. doi: 10.5114/pg.2014.47501. Epub 2015 Jan 14. Prz Gastroenterol. 2015. PMID: 25960807 Free PMC article. Review.
References
-
- Elias E. Liver failure and liver disease. Hepatology. 2006;43:S239–S242. - PubMed
-
- Davis CL, Gonwa TA, Wilkinson AH. Identification of patients best suited for combined liver-kidney transplantation: part II. Liver Transpl. 2002;8:193–211. - PubMed
-
- Han MK, Hyzy R. Advances in critical care management of hepatic failure and insufficiency. Crit Care Med. 2006;34:S225–S231. - PubMed
-
- Blei AT. Selection for acute liver failure: have we got it right? Liver Transpl. 2005;11:S30–S34. - PubMed
-
- Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

