Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study
- PMID: 19249413
- PMCID: PMC2671287
- DOI: 10.1016/j.ahj.2008.11.018
Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study
Abstract
Background: Selection of antihypertensive therapy is often empiric, and use of genetic information to guide drug therapy selection holds future promise.
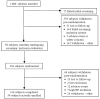
Trial design: The objective of this trial is to identify the genetic determinants of the antihypertensive and adverse metabolic responses to a thiazide diuretic (hydrochlorothiazide), a beta-blocker (atenolol), and their combination. This will be accomplished through candidate gene and genome-wide association approaches. Individuals with uncomplicated hypertension (N = 800), with ages 17 and 65 years, are being enrolled. Current antihypertensive therapy is discontinued, and hypertension is confirmed, along with collection of other baseline data. Subjects are then randomized to either hydrochlorothiazide or atenolol, with 1 dose titration step, followed by assessment of response to therapy after at least 6 weeks on the target dose. Those with blood pressure >120/70 mm Hg have the second drug added, with similar dose titration and response assessment procedures. Data collected include home, office, and 24-hour ambulatory blood pressure. Biological samples collected in the fasting state include plasma, serum, DNA (buffy coat), and urine. Epstein-Barr virus transformed lymphocyte cell lines are also being created.
Conclusions: Pharmacogenetic-guided therapy holds clinical potential for hypertension, but the literature in the field is limited. This trial will add substantially to our understanding of the genetic determinants of antihypertensive and adverse metabolic responses to 2 commonly used antihypertensive drug classes.
Trial registration: ClinicalTrials.gov NCT00246519.
Figures
References
-
- AHA. Heart Disease and Stroke Statistics - 2008 Update. Dallas, Texas: American Heart Assocation; 2008.
-
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–2571. - PubMed
-
- Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706–15. discussion 2715. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases