Alternating access in maltose transporter mediated by rigid-body rotations
- PMID: 19250913
- PMCID: PMC2714826
- DOI: 10.1016/j.molcel.2009.01.035
Alternating access in maltose transporter mediated by rigid-body rotations
Abstract
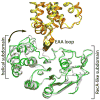
ATP-binding cassette transporters couple ATP hydrolysis to substrate translocation through an alternating access mechanism, but the nature of the conformational changes in a transport cycle remains elusive. Previously we reported the structure of the maltose transporter MalFGK(2) in an outward-facing conformation in which the transmembrane (TM) helices outline a substrate-binding pocket open toward the periplasmic surface and ATP is poised for hydrolysis along the closed nucleotide-binding dimer interface. Here we report the structure of the nucleotide-free maltose transporter in which the substrate binding pocket is only accessible from the cytoplasm and the nucleotide-binding interface is open. Comparison of the same transporter crystallized in two different conformations reveals that alternating access involves rigid-body rotations of the TM subdomains that are coupled to the closure and opening of the nucleotide-binding domain interface. The comparison also reveals that point mutations enabling binding protein-independent transport line dynamic interfaces in the TM region.
Figures
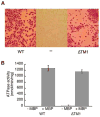

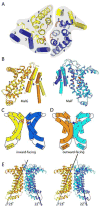
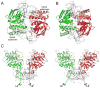
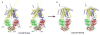
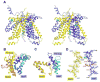
References
-
- Abrahams JP, Leslie AG. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr D Biol Crystallogr. 1996;52:30–42. - PubMed
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
-
- Austermuhle MI, Hall JA, Klug CS, Davidson AL. Maltose-binding protein is open in the catalytic transition state for ATP hydrolysis during maltose transport. J Biol Chem. 2004;279:28243–28250. - PubMed
-
- Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. - PubMed
-
- Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell. 2003;12:651–661. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

