Deleting the accessory subunit KChIP2 results in loss of I(to,f) and increased I(K,slow) that maintains normal action potential configuration
- PMID: 19251214
- PMCID: PMC2731656
- DOI: 10.1016/j.hrthm.2008.11.023
Deleting the accessory subunit KChIP2 results in loss of I(to,f) and increased I(K,slow) that maintains normal action potential configuration
Abstract
Background: Four voltage-gated potassium currents, I(to,f) (K(V)4.2), I(to,s) (K(V)1.4), I(K,slow) (K(V)1.5+K(V)2.1), and I(SS) (TASK1), govern murine ventricular repolarization. Although the accessory subunit KChIP2 influences I(to,f) expression, in preliminary experiments we found that action potential duration (APD) is maintained in KChIP2 knockout mice.
Objective: We tested the role of KChIP2 in regulating APD and studied the underlying ionic currents.
Methods: We used microelectrode techniques, whole-cell patch clamp studies, and real-time polymerase chain reaction amplification to characterize ventricular repolarization and its determinants in wild-type and KChIP2(-/-) mice.
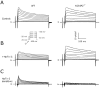
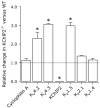
Results: Despite comparable baseline action potentials, APD was more markedly prolonged by 4-aminopyridine (4-AP) in KChIP2(-/-) preparations. Peak K(+) current densities were similar in wild-type and KChIP2(-/-) cells (mean +/- SEM I(P): 28.3 +/- 2 (n = 27) vs. 29.2 +/- 2 pA/pF (n = 24), respectively; P > .05). Heteropodatoxin-2 (HpTx-2, 1 microM) had no effect on current amplitude in KChIP2(-/-) myocytes. The current fractions sensitive to 4-AP (50 microM and 1 mM) were larger in KChIP2(-/-) than wild-type (P < .05). Real-time polymerase chain reaction showed absence of KChIP2 and increased K(V)1.5 expression in KChIP2(-/-) ventricular myocardium.
Conclusion: KChIP2 deficiency eliminated HpTx-2-sensitive I(to,f), but had little impact on total APD, secondary to upregulation of 4-AP-sensitive I(K,slow) in association with increased K(V)1.5 expression. There is increased sensitivity to 4-AP-mediated APD prolongation in KChIP2(-/-). Thus, KChIP2 seems important for murine repolarization in circumstances of reduced repolarization reserve.
Figures




Similar articles
-
Stabilization of Kv4 protein by the accessory K(+) channel interacting protein 2 (KChIP2) subunit is required for the generation of native myocardial fast transient outward K(+) currents.J Physiol. 2013 Sep 1;591(17):4149-66. doi: 10.1113/jphysiol.2013.255836. Epub 2013 May 27. J Physiol. 2013. PMID: 23713033 Free PMC article.
-
Loss of K+ currents in heart failure is accentuated in KChIP2 deficient mice.J Cardiovasc Electrophysiol. 2014 Aug;25(8):896-904. doi: 10.1111/jce.12422. Epub 2014 May 2. J Cardiovasc Electrophysiol. 2014. PMID: 24678923
-
Kv4.3-Encoded Fast Transient Outward Current Is Presented in Kv4.2 Knockout Mouse Cardiomyocytes.PLoS One. 2015 Jul 21;10(7):e0133274. doi: 10.1371/journal.pone.0133274. eCollection 2015. PLoS One. 2015. PMID: 26196737 Free PMC article.
-
Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes.Circ Res. 2005 Mar 4;96(4):451-8. doi: 10.1161/01.RES.0000156890.25876.63. Epub 2005 Jan 20. Circ Res. 2005. PMID: 15662035
-
Myocardial KChIP2 Expression in Guinea Pig Resolves an Expanded Electrophysiologic Role.PLoS One. 2016 Jan 14;11(1):e0146561. doi: 10.1371/journal.pone.0146561. eCollection 2016. PLoS One. 2016. PMID: 26764482 Free PMC article.
Cited by
-
Why T waves change: a reminiscence and essay.Heart Rhythm. 2009 Nov;6(11 Suppl):S56-61. doi: 10.1016/j.hrthm.2009.07.022. Heart Rhythm. 2009. PMID: 19880075 Free PMC article.
-
KV Channel-Interacting Proteins in the Neurological and Cardiovascular Systems: An Updated Review.Cells. 2023 Jul 20;12(14):1894. doi: 10.3390/cells12141894. Cells. 2023. PMID: 37508558 Free PMC article. Review.
-
KChIP2 genotype dependence of transient outward current (Ito) properties in cardiomyocytes isolated from male and female mice.PLoS One. 2017 Jan 31;12(1):e0171213. doi: 10.1371/journal.pone.0171213. eCollection 2017. PLoS One. 2017. PMID: 28141821 Free PMC article.
-
Transcriptional and electrophysiological consequences of KChIP2-mediated regulation of CaV1.2.Channels (Austin). 2009 Sep-Oct;3(5):308-10. doi: 10.4161/chan.3.5.9560. Epub 2009 Sep 16. Channels (Austin). 2009. PMID: 19713767 Free PMC article.
-
Impact of KChIP2 on Cardiac Electrophysiology and the Progression of Heart Failure.Front Physiol. 2012 May 4;3:118. doi: 10.3389/fphys.2012.00118. eCollection 2012. Front Physiol. 2012. PMID: 22586403 Free PMC article.
References
-
- Kaab S, Dixon J, Duc J, et al. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98(14):1383–1393. - PubMed
-
- Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85(3):1175–1188. - PubMed
-
- Huang B, Qin D, El-Sherif N. Spatial alterations of Kv channels expression and K(+) currents in post-MI remodeled rat heart. Cardiovasc Res. 2001;52(2):246–254. - PubMed
-
- Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

