Deleting the accessory subunit KChIP2 results in loss of I(to,f) and increased I(K,slow) that maintains normal action potential configuration
- PMID: 19251214
- PMCID: PMC2731656
- DOI: 10.1016/j.hrthm.2008.11.023
Deleting the accessory subunit KChIP2 results in loss of I(to,f) and increased I(K,slow) that maintains normal action potential configuration
Abstract
Background: Four voltage-gated potassium currents, I(to,f) (K(V)4.2), I(to,s) (K(V)1.4), I(K,slow) (K(V)1.5+K(V)2.1), and I(SS) (TASK1), govern murine ventricular repolarization. Although the accessory subunit KChIP2 influences I(to,f) expression, in preliminary experiments we found that action potential duration (APD) is maintained in KChIP2 knockout mice.
Objective: We tested the role of KChIP2 in regulating APD and studied the underlying ionic currents.
Methods: We used microelectrode techniques, whole-cell patch clamp studies, and real-time polymerase chain reaction amplification to characterize ventricular repolarization and its determinants in wild-type and KChIP2(-/-) mice.
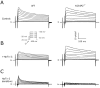
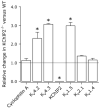
Results: Despite comparable baseline action potentials, APD was more markedly prolonged by 4-aminopyridine (4-AP) in KChIP2(-/-) preparations. Peak K(+) current densities were similar in wild-type and KChIP2(-/-) cells (mean +/- SEM I(P): 28.3 +/- 2 (n = 27) vs. 29.2 +/- 2 pA/pF (n = 24), respectively; P > .05). Heteropodatoxin-2 (HpTx-2, 1 microM) had no effect on current amplitude in KChIP2(-/-) myocytes. The current fractions sensitive to 4-AP (50 microM and 1 mM) were larger in KChIP2(-/-) than wild-type (P < .05). Real-time polymerase chain reaction showed absence of KChIP2 and increased K(V)1.5 expression in KChIP2(-/-) ventricular myocardium.
Conclusion: KChIP2 deficiency eliminated HpTx-2-sensitive I(to,f), but had little impact on total APD, secondary to upregulation of 4-AP-sensitive I(K,slow) in association with increased K(V)1.5 expression. There is increased sensitivity to 4-AP-mediated APD prolongation in KChIP2(-/-). Thus, KChIP2 seems important for murine repolarization in circumstances of reduced repolarization reserve.
Figures




References
-
- Kaab S, Dixon J, Duc J, et al. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98(14):1383–1393. - PubMed
-
- Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85(3):1175–1188. - PubMed
-
- Huang B, Qin D, El-Sherif N. Spatial alterations of Kv channels expression and K(+) currents in post-MI remodeled rat heart. Cardiovasc Res. 2001;52(2):246–254. - PubMed
-
- Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

