Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice
- PMID: 19251629
- PMCID: PMC2884172
- DOI: 10.1126/science.1169096
Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice
Abstract
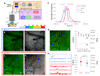
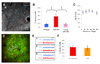
Although senile plaques focally disrupt neuronal health, the functional response of astrocytes to Alzheimer's disease pathology is unknown. Using multiphoton fluorescence lifetime imaging microscopy in vivo, we quantitatively imaged astrocytic calcium homeostasis in a mouse model of Alzheimer's disease. Resting calcium was globally elevated in the astrocytic network, but was independent of proximity to individual plaques. Time-lapse imaging revealed that calcium transients in astrocytes were more frequent, synchronously coordinated across long distances, and uncoupled from neuronal activity. Furthermore, rare intercellular calcium waves were observed, but only in mice with amyloid-beta plaques, originating near plaques and spreading radially at least 200 micrometers. Thus, although neurotoxicity is observed near amyloid-beta deposits, there exists a more general astrocyte-based network response to focal pathology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

