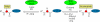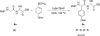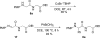Site-specific C-functionalization of free-(NH) peptides and glycine derivatives via direct C-H bond functionalization
- PMID: 19251635
- PMCID: PMC2649206
- DOI: 10.1073/pnas.0809052106
Site-specific C-functionalization of free-(NH) peptides and glycine derivatives via direct C-H bond functionalization
Abstract
A copper-catalyzed alpha-functionalization of glycine derivatives and short peptides with nucleophiles is described. The present method provides ways to introduce functionalities such as aryl, vinyl, alkynyl, or indolyl specifically to the terminal glycine moieties of small peptides, which are normally difficult in peptide modifications. Furthermore, on functionalization, the configurations of other stereocenters in the peptides could be maintained. Because the functionalized peptides could easily be deprotected and carried onto the next coupling process, our approach provides a useful tool for the peptide-based biological research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Beak P, Zajdel WJ, Reitz DB. Metalation and electrophilic substitution of amine derivatives adjacent to nitrogen: α-metallo amine synthetic equivalents. Chem Rev. 1984;84:471–523.
-
- Meyers AI. Formamidines as precursors to α-amino carbanions and their application to asymmetric carbon-carbon bond-forming reactions. Aldrichim Acta. 1985;18:59–68.
-
- Maruoka K, Ooi T. Enantioselective amino acid synthesis by chiral phase-transfer catalysis. Chem Rev. 2003;103:3013–3028. - PubMed
-
- Hashimoto T, Maruoka K. Recent development and application of chiral phase- transfer catalysts. Chem Rev. 2007;107:5656–5682. - PubMed
-
- Easton CJ, Scharfbillig IM, Tan EW. Selective modification of glycine residues in dipeptides. Tetrahedron Lett. 1988;29:1565–1568.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources