Distinct modes of gene regulation by a cell-specific transcriptional activator
- PMID: 19251649
- PMCID: PMC2657397
- DOI: 10.1073/pnas.0808347106
Distinct modes of gene regulation by a cell-specific transcriptional activator
Abstract
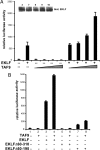
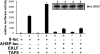
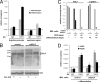
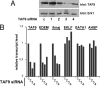
The architectural layout of a eukaryotic RNA polymerase II core promoter plays a role in general transcriptional activation. However, its role in tissue-specific expression is not known. For example, differing modes of its recognition by general transcription machinery can provide an additional layer of control within which a single tissue-restricted transcription factor may operate. Erythroid Kruppel-like factor (EKLF) is a hematopoietic-specific transcription factor that is critical for the activation of subset of erythroid genes. We find that EKLF interacts with TATA binding protein-associated factor 9 (TAF9), which leads to important consequences for expression of adult beta-globin. First, TAF9 functionally supports EKLF activity by enhancing its ability to activate the beta-globin gene. Second, TAF9 interacts with a conserved beta-globin downstream promoter element, and ablation of this interaction by beta-thalassemia-causing mutations decreases its promoter activity and disables superactivation. Third, depletion of EKLF prevents recruitment of TAF9 to the beta-globin promoter, whereas depletion of TAF9 drastically impairs beta-promoter activity. However, a TAF9-independent mode of EKLF transcriptional activation is exhibited by the alpha-hemoglobin-stabilizing protein (AHSP) gene, which does not contain a discernable downstream promoter element. In this case, TAF9 does not enhance EKLF activity and depletion of TAF9 has no effect on AHSP promoter activation. These studies demonstrate that EKLF directs different modes of tissue-specific transcriptional activation depending on the architecture of its target core promoter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. - PubMed
-
- Muller F, Demeny MA, Tora L. New problems in RNA polymerase II transcription initiation: Matching the diversity of core promoters with a variety of promoter recognition factors. J Biol Chem. 2007;282:14685–14689. - PubMed
-
- Holstege FC, et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. - PubMed
-
- Kuras L, Kosa P, Mencia M, Struhl K. TAF-containing and TAF-independent forms of transcriptionally active TBP in vivo. Science. 2000;288:1244–1248. - PubMed
-
- Green MR. TBP-associated factors (TAFIIs): Multiple, selective transcriptional mediators in common complexes. Trends Biochem Sci. 2000;25:59–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

