Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds
- PMID: 19251665
- PMCID: PMC2657427
- DOI: 10.1073/pnas.0809080106
Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds
Abstract
The expression of ASPP2 (53BP2L), a proapoptotic member of a family of p53-binding proteins, is frequently suppressed in many human cancers. Accumulating evidence suggests that ASPP2 inhibits tumor growth; however, the mechanisms by which ASPP2 suppresses tumor formation remain to be clarified. To study this, we targeted the ASPP2 allele in a mouse by replacing exons 10-17 with a neoR gene. ASPP2(-/-) mice were not viable because of an early embryonic lethal event. Although ASPP2(+/-) mice appeared developmentally normal, they displayed an increased incidence of a variety of spontaneous tumors as they aged. Moreover, gamma-irradiated 6-week-old ASPP2(+/-) mice developed an increased incidence of high-grade T cell lymphomas of thymic origin compared with ASPP2(+/+) mice. Primary thymocytes derived from ASPP2(+/-) mice exhibited an attenuated apoptotic response to gamma-irradiation compared with ASPP2(+/+) thymocytes. Additionally, ASPP2(+/-) primary mouse embryonic fibroblasts demonstrated a defective G(0)/G(1) cell cycle checkpoint after gamma-irradiation. Our results demonstrate that ASPP2 is a haploinsufficient tumor suppressor and, importantly, open new avenues for investigation into the mechanisms by which disruption of ASPP2 pathways could play a role in tumorigenesis and response to therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


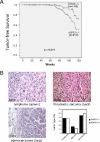




References
Publication types
MeSH terms
Substances
Grants and funding
- 5-P30-CA69533/CA/NCI NIH HHS/United States
- CA89305/CA/NCI NIH HHS/United States
- K08 CA085773/CA/NCI NIH HHS/United States
- R01 CA076316/CA/NCI NIH HHS/United States
- P01 CA034233/CA/NCI NIH HHS/United States
- R01 CA104997/CA/NCI NIH HHS/United States
- CA104997/CA/NCI NIH HHS/United States
- R01 CA089305/CA/NCI NIH HHS/United States
- R01 HL069133/HL/NHLBI NIH HHS/United States
- CA076316/CA/NCI NIH HHS/United States
- P30 CA069533/CA/NCI NIH HHS/United States
- R01 HL077818/HL/NHLBI NIH HHS/United States
- HL069133/HL/NHLBI NIH HHS/United States
- HL077818/HL/NHLBI NIH HHS/United States
- CA85773/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

