A plant-specific RNA-binding domain revealed through analysis of chloroplast group II intron splicing
- PMID: 19251672
- PMCID: PMC2657376
- DOI: 10.1073/pnas.0812503106
A plant-specific RNA-binding domain revealed through analysis of chloroplast group II intron splicing
Abstract
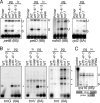
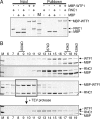
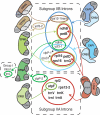
Comparative genomics has provided evidence for numerous conserved protein domains whose functions remain unknown. We identified a protein harboring "domain of unknown function 860" (DUF860) as a component of group II intron ribonucleoprotein particles in maize chloroplasts. This protein, assigned the name WTF1 ("what's this factor?"), coimmunoprecipitates from chloroplast extract with group II intron RNAs, is required for the splicing of the introns with which it associates, and promotes splicing in the context of a heterodimer with the RNase III-domain protein RNC1. Both WTF1 and its resident DUF860 bind RNA in vitro, demonstrating that DUF860 is a previously unrecognized RNA-binding domain. DUF860 is found only in plants, where it is represented in a protein family comprising 14 orthologous groups in angiosperms. Most members of the DUF860 family are predicted to localize to chloroplasts or mitochondria, suggesting that proteins with this domain have multiple roles in RNA metabolism in both organelles. These findings add to emerging evidence that the coevolution of nuclear and organellar genomes spurred the evolution of diverse noncanonical RNA-binding motifs that perform organelle-specific functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
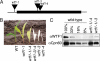



References
-
- Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 2004;5:123–135. - PubMed
-
- Marchfelder A, Binder S. In: Molecular Biology and Biotechnology of Plant Organelles. Daniell H, Chase C, editors. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2004. pp. 261–294.
-
- Zerges W. In: Molecular Biology and Biotechnology of Plant Organelles. Daniell H, Chase C, editors. Dordrecth, The Netherlands: Springer; 2004. pp. 347–383.
-
- Bollenbach TJ, Schuster G, Stern DB. Cooperation of endo- and exoribonucleases in chloroplast mRNA turnover. Prog Nucleic Acid Res Mol Biol. 2004;78:305–337. - PubMed
-
- Schmitz-Linneweber C, Barkan A. In: Cell and Molecular Biology of Plastids. Bock R, editor. Vol 19. Berlin and Heidelberg: Springer; 2007. pp. 213–248.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

