Pyruvate kinase-deficient Escherichia coli exhibits increased plasmid copy number and cyclic AMP levels
- PMID: 19251844
- PMCID: PMC2681811
- DOI: 10.1128/JB.01422-08
Pyruvate kinase-deficient Escherichia coli exhibits increased plasmid copy number and cyclic AMP levels
Abstract
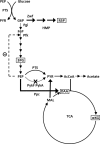
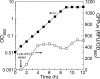
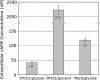
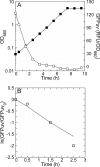
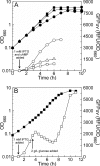
Previously established consequences of abolishing pyruvate kinase (Pyk) activity in Escherichia coli during aerobic growth on glucose include reduced acetate production, elevated hexose monophosphate (HMP) pathway flux, elevated phosphoenolpyruvate carboxylase (Ppc) flux, and an increased ratio of phosphoenolpyruvate (PEP) to pyruvate. These traits inspired two hypotheses. First, the mutant (PB25) may maintain more plasmid than the wild type (JM101) by combining traits reported to facilitate plasmid DNA synthesis (i.e., decreased Pyk flux and increased HMP pathway and Ppc fluxes). Second, PB25 likely possesses a higher level of cyclic AMP (cAMP) than JM101. This is based on reports that connect elevated PEP/pyruvate ratios to phosphotransferase system signaling and adenylate cyclase activation. To test the first hypothesis, the strains were transformed with a pUC-based, high-copy-number plasmid (pGFPuv), and copy numbers were measured. PB25 exhibited a fourfold-higher copy number than JM101 when grown at 37 degrees C. At 42 degrees C, its plasmid content was ninefold higher than JM101 at 37 degrees C. To test the second hypothesis, cAMP was measured, and the results confirmed it to be higher in PB25 than JM101. This elevation was not enough to elicit a strong regulatory effect, however, as indicated by the comparative expression of the pGFPuv-based reporter gene, gfp(uv), under the control of the cAMP-responsive lac promoter. The elevated cAMP in PB25 suggests that Pyk may participate in glucose catabolite repression by serving among all of the factors that tighten gene expression.
Figures
References
-
- Arditti, R. R., J. G. Scaife, and J. R. Beckwith. 1968. The nature of mutants in the lac promoter region. J. Mol. Biol. 38421-426. - PubMed
-
- Birnbaum, S., and J. E. Bailey. 1991. Plasmid presence changes the relative levels of many host cell proteins and ribosome components in recombinant Escherichia coli. Biotechnol. Bioeng. 37736-745. - PubMed
-
- Blangy, D., H. Buc, and J. Monod. 1968. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J. Mol. Biol. 3113-35. - PubMed
-
- Cox, R. A. 2004. Quantitative relationships for specific growth rates and macromolecular compositions of Mycobacterium tuberculosis, Streptomyces coelicolor A3(2) and Escherichia coli B/r: an integrative theoretical approach. Microbiology 1501413-1426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

