Antibody-mediated immobilization of Cryptococcus neoformans promotes biofilm formation
- PMID: 19251903
- PMCID: PMC2675221
- DOI: 10.1128/AEM.02846-08
Antibody-mediated immobilization of Cryptococcus neoformans promotes biofilm formation
Abstract
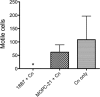
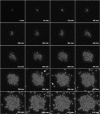
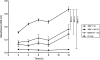
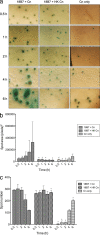
Most microbes, including the fungal pathogen Cryptococcus neoformans, can grow as biofilms. Biofilms confer upon microbes a range of characteristics, including an ability to colonize materials such as shunts and catheters and increased resistance to antibiotics. Here, we provide evidence that coating surfaces with a monoclonal antibody to glucuronoxylomannan, the major component of the fungal capsular polysaccharide, immobilizes cryptococcal cells to a surface support and, subsequently, promotes biofilm formation. We used time-lapse microscopy to visualize the growth of cryptococcal biofilms, generating the first movies of fungal biofilm growth. We show that when fungal cells are immobilized using surface-attached specific antibody to the capsule, the initial stages of biofilm formation are significantly faster than those on surfaces with no antibody coating or surfaces coated with unspecific monoclonal antibody. Time-lapse microscopy revealed that biofilm growth was a dynamic process in which cells shuffled position during budding and was accompanied by emergence of planktonic variant cells that left the attached biofilm community. The planktonic variant cells exhibited mobility, presumably by Brownian motion. Our results indicate that microbial immobilization by antibody capture hastens biofilm formation and suggest that antibody coating of medical devices with immunoglobulins must exclude binding to common pathogenic microbes and the possibility that this effect could be exploited in industrial microbiology.
Figures

Similar articles
-
Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy.Infect Immun. 2005 Oct;73(10):6350-62. doi: 10.1128/IAI.73.10.6350-6362.2005. Infect Immun. 2005. PMID: 16177306 Free PMC article.
-
EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans.Appl Environ Microbiol. 2012 Nov;78(22):7977-84. doi: 10.1128/AEM.01953-12. Epub 2012 Aug 31. Appl Environ Microbiol. 2012. PMID: 22941091 Free PMC article.
-
Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen.Infect Immun. 2004 Jun;72(6):3674-9. doi: 10.1128/IAI.72.6.3674-3679.2004. Infect Immun. 2004. PMID: 15155683 Free PMC article.
-
Mechanism of action of antibody to capsular polysaccharide in Cryptococcus neoformans infection.Front Biosci. 1998 Feb 1;3:d136-51. doi: 10.2741/a270. Front Biosci. 1998. PMID: 9445465 Review.
-
Capsular polysaccharides of Cryptococcus neoformans.Rev Infect Dis. 1984 Sep-Oct;6(5):619-24. doi: 10.1093/clinids/6.5.619. Rev Infect Dis. 1984. PMID: 6209768 Review.
Cited by
-
Antifungal and Antibiofilm In Vitro Activities of Ursolic Acid on Cryptococcus neoformans.Curr Microbiol. 2022 Aug 16;79(10):293. doi: 10.1007/s00284-022-02992-5. Curr Microbiol. 2022. PMID: 35972650
-
The Hidden Fortress: A Comprehensive Review of Fungal Biofilms with Emphasis on Cryptococcus neoformans.J Fungi (Basel). 2025 Mar 19;11(3):236. doi: 10.3390/jof11030236. J Fungi (Basel). 2025. PMID: 40137272 Free PMC article. Review.
-
Fungal cell wall dynamics and infection site microenvironments: signal integration and infection outcome.Curr Opin Microbiol. 2013 Aug;16(4):385-90. doi: 10.1016/j.mib.2013.03.003. Epub 2013 Apr 15. Curr Opin Microbiol. 2013. PMID: 23597789 Free PMC article.
-
Virulence of Cryptococcus sp. Biofilms In Vitro and In Vivo using Galleria mellonella as an Alternative Model.Front Microbiol. 2016 Mar 9;7:290. doi: 10.3389/fmicb.2016.00290. eCollection 2016. Front Microbiol. 2016. PMID: 27014214 Free PMC article.
-
Cryptococcal Traits Mediating Adherence to Biotic and Abiotic Surfaces.J Fungi (Basel). 2018 Jul 29;4(3):88. doi: 10.3390/jof4030088. J Fungi (Basel). 2018. PMID: 30060601 Free PMC article. Review.
References
-
- Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. - PubMed
-
- Anderson, G. G., and G. A. O'Toole. 2008. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 322:85-105. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

