Different ways to die: cell death modes of the unicellular chlorophyte Dunaliella viridis exposed to various environmental stresses are mediated by the caspase-like activity DEVDase
- PMID: 19251986
- PMCID: PMC2652065
- DOI: 10.1093/jxb/ern330
Different ways to die: cell death modes of the unicellular chlorophyte Dunaliella viridis exposed to various environmental stresses are mediated by the caspase-like activity DEVDase
Abstract
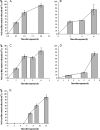
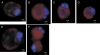
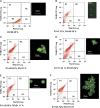
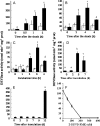
Programmed cell death is necessary for homeostasis in multicellular organisms and it is also widely recognized to occur in unicellular organisms. However, the mechanisms through which it occurs in unicells, and the enzymes involved within the final response is still the subject of heated debate. It is shown here that exposure of the unicellular microalga Dunaliella viridis to several environmental stresses, induced different cell death morphotypes, depending on the stimulus received. Senescent cells demonstrated classical and unambiguous apoptotic-like characteristics such as chromatin condensation, DNA fragmentation, intact organelles, and blebbing of the cell membrane. Acute heat shock caused general swelling and altered plasma membrane, but the presence of chromatin clusters and DNA strand breaks suggested a necrotic-like event. UV irradiated cells presented changes typical for necrosis, together with apoptotic characteristics resembling an intermediate cell-death phenotype termed aponecrosis-like. Cells subjected to hyperosmotic shock revealed chromatin spotting without DNA fragmentation, and extensive cytoplasmic swelling and vacuolization, comparable to a paraptotic-like cell death phenotype. Nitrogen-starved cells showed pyknosis, blebbing, and cytoplasmic consumption, indicating a similarity to autophagic/vacuolar-like cell death. The caspase-like activity DEVDase was measured by using the fluorescent substrate Ac-DEVD-AMC and antibodies against the human caspase-3 active enzyme cross-reacted with bands, the intensity of which paralleled the activity. All the environmental stresses tested produced a substantial increase in both DEVDase activity and protein levels. The irreversible caspase-3 inhibitor Z-DEVD-FMK completely inhibited the enzymatic activity whereas serine and aspartyl proteases inhibitors did not. These results show that cell death in D. viridis does not conform to a single pattern and that environmental stimuli may produce different types of cell death depending on the type and intensity of the stimulus, all of which help to understand the cell death-dependent and cell death-independent functions of caspase-like proteins. Hence, these data support the theory that alternative, non-apoptotic programmed cell death (PCDs), exist either in parallel or in an independent manner with apoptosis and were already present in single-celled organisms that evolved some 1.2-1.6 billion years ago.
Figures


References
-
- Aigner T. Apoptosis, necrosis, or whatever: how to find out what really happens? Journal of Pathology. 2002;198:1–4. - PubMed
-
- Algeciras-Schimnich A, Barnhart BC, Peter ME. Apoptosis: independent functions of killer caspases. Current Opinion in Cell Biology. 2002;14:721–726. - PubMed
-
- Ameisen JC. The origin of programmed cell death. Science. 1996;272:1278–1279. - PubMed
-
- Ameisen JC. The evolutionary origin and role of programmed cell death in single celled organisms: a new view of executioners, mitochondria, host–pathogen interactions, and the the role of death in the process of natural selection. In: Lockshin R, Zakeri Z, Tilly J, editors. When cells die. New York: Wiley-Liss; 1998. pp. 3–56.
-
- Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death and Differentiation. 2002;9:367–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

