Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans
- PMID: 19252089
- PMCID: PMC2685344
- DOI: 10.1152/ajpheart.01226.2008
Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans
Abstract
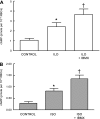
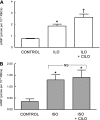
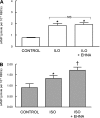
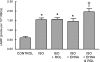
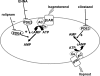
Activation of the G protein G(s) results in increases in cAMP, a necessary step in the pathway for ATP release from rabbit and human erythrocytes. In all cells, the level of cAMP is the product of its synthesis by adenylyl cyclase and its hydrolysis by phosphodiesterases (PDEs). Both iloprost (Ilo), a PGI(2) analog, and isoproterenol (Iso), a beta-agonist, stimulate receptor-mediated increases in cAMP in rabbit and human erythrocytes. However, the specific PDEs associated with each of these signaling pathways in the erythrocyte have not been fully characterized. Previously, we reported that PDE3B is present in rabbit and human erythrocyte membranes and that PDE3 inhibitors potentiate Ilo-induced increases in cAMP. Here we report that inhibitors of either PDE2 or PDE4, erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) and rolipram, respectively, potentiate Iso-induced increases in cAMP in rabbit and human erythrocytes. Importantly, these inhibitors had no effect on cAMP increases associated with the incubation of erythrocytes with Ilo. In addition, we establish, for the first time, the presence of PDE2A protein in rabbit and human erythrocyte membranes. Finally, we determined that preincubation of human erythrocytes with EHNA and rolipram together potentiate Iso-induced ATP release, whereas preincubation with cilostazol enhances Ilo-induced release of ATP. These results are consistent with the hypothesis that, in rabbit and human erythrocytes, Ilo-induced increases in cAMP and ATP release are regulated by PDE3, whereas those associated with Iso are regulated by the activities of both PDE2 and PDE4. These studies demonstrate that PDE activity in these cells is localized to specific signaling pathways.
Figures


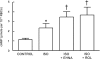


References
-
- Babu CR, Azhar S, Krishna Murti CR. Loss of epinephrine stimulated synthesis of cyclic adenosine 3′:5′ monophosphate during maturation of rabbit and human reticulocytes. Med Biol 53: 148–155, 1975. - PubMed
-
- Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett 579: 3264–3270, 2005. - PubMed
-
- Beltman J, Becker DE, Butt E, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA. Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: topology of the catalytic domains. Mol Pharmacol 47: 330–339, 1995. - PubMed
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006. - PubMed
-
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

