Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes
- PMID: 19252095
- PMCID: PMC2685326
- DOI: 10.1152/ajpheart.00864.2008
Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes
Abstract
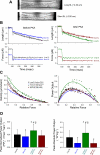
The Frank-Starling relationship of the heart yields increased stroke volume with greater end-diastolic volume, and this relationship is steeper after beta-adrenergic stimulation. The underlying basis for the Frank-Starling mechanism involves length-dependent changes in both Ca(2+) sensitivity of myofibrillar force and power output. In this study, we tested the hypothesis that PKA-induced phosphorylation of myofibrillar proteins would increase the length dependence of myofibrillar power output, which would provide a myofibrillar basis to, in part, explain the steeper Frank-Starling relations after beta-adrenergic stimulation. For these experiments, adult rat left ventricles were mechanically disrupted, permeabilized cardiac myocyte preparations were attached between a force transducer and position motor, and the length dependence of loaded shortening and power output were measured before and after treatment with PKA. PKA increased the phosphorylation of myosin binding protein C and cardiac troponin I, as assessed by autoradiography. In terms of myocyte mechanics, PKA decreased the Ca(2+) sensitivity of force and increased loaded shortening and power output at all relative loads when the myocyte preparations were at long sarcomere length ( approximately 2.30 mum). PKA had less of an effect on loaded shortening and power output at short sarcomere length ( approximately 2.0 mum). These changes resulted in a greater length dependence of myocyte power output after PKA treatment; peak normalized power output increased approximately 20% with length before PKA and approximately 40% after PKA. These results suggest that PKA-induced phosphorylation of myofibrillar proteins explains, in part, the steeper ventricular function curves (i.e., Frank-Starling relationship) after beta-adrenergic stimulation of the left ventricle.
Figures


Similar articles
-
Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes.Circ Res. 2001 Dec 7;89(12):1184-90. doi: 10.1161/hh2401.101908. Circ Res. 2001. PMID: 11739284
-
Molecule specific effects of PKA-mediated phosphorylation on rat isolated heart and cardiac myofibrillar function.Arch Biochem Biophys. 2016 Jul 1;601:22-31. doi: 10.1016/j.abb.2016.01.019. Epub 2016 Feb 15. Arch Biochem Biophys. 2016. PMID: 26854722 Free PMC article.
-
Cardiac Myosin-binding Protein C and Troponin-I Phosphorylation Independently Modulate Myofilament Length-dependent Activation.J Biol Chem. 2015 Dec 4;290(49):29241-9. doi: 10.1074/jbc.M115.686790. Epub 2015 Oct 9. J Biol Chem. 2015. PMID: 26453301 Free PMC article.
-
Cardiac function and modulation of sarcomeric function by length.Cardiovasc Res. 2008 Mar 1;77(4):627-36. doi: 10.1093/cvr/cvm099. Epub 2007 Dec 12. Cardiovasc Res. 2008. PMID: 18079105 Review.
-
The interdependence of Ca2+ activation, sarcomere length, and power output in the heart.Pflugers Arch. 2011 Jul;462(1):61-7. doi: 10.1007/s00424-011-0949-y. Epub 2011 Mar 15. Pflugers Arch. 2011. PMID: 21404040 Free PMC article. Review.
Cited by
-
Titin-mediated control of cardiac myofibrillar function.Arch Biochem Biophys. 2014 Jun 15;552-553:83-91. doi: 10.1016/j.abb.2013.11.005. Epub 2013 Nov 20. Arch Biochem Biophys. 2014. PMID: 24269766 Free PMC article.
-
Regulation of Myofilament Contractile Function in Human Donor and Failing Hearts.Front Physiol. 2020 May 25;11:468. doi: 10.3389/fphys.2020.00468. eCollection 2020. Front Physiol. 2020. PMID: 32523542 Free PMC article.
-
A Novel "Cut and Paste" Method for In Situ Replacement of cMyBP-C Reveals a New Role for cMyBP-C in the Regulation of Contractile Oscillations.Circ Res. 2020 Mar 13;126(6):737-749. doi: 10.1161/CIRCRESAHA.119.315760. Epub 2020 Feb 13. Circ Res. 2020. PMID: 32078438 Free PMC article.
-
The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium.Am J Physiol Heart Circ Physiol. 2015 Dec 15;309(12):H2077-86. doi: 10.1152/ajpheart.00685.2015. Epub 2015 Oct 9. Am J Physiol Heart Circ Physiol. 2015. PMID: 26453335 Free PMC article.
-
G protein, phosphorylated-GATA4 and VEGF expression in the hearts of transgenic mice overexpressing β1- and β2-adrenergic receptors.Mol Med Rep. 2017 Jun;15(6):4049-4054. doi: 10.3892/mmr.2017.6526. Epub 2017 Apr 28. Mol Med Rep. 2017. PMID: 28487987 Free PMC article.
References
-
- Bristow M, Ginsburg R, Minobe W, Cubicciotti R, Sageman W, Billingham M, Harrison D, Stinson E. Decrease catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982. - PubMed
-
- Chase PB, MacPherson M, Daniel TL. A spatially explicit nanomechanical model of the half sarcomere: myofilament compliance affects calcium activation. Ann Biomed Eng 32: 1559–1568, 2004. - PubMed
-
- Endoh M, Blinks JR. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through α- and β-adrenoceptors. Circ Res 62: 247–265, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

