Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever
- PMID: 19252743
- PMCID: PMC2646144
- DOI: 10.1371/journal.pone.0004666
Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever
Abstract
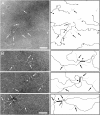
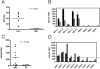
Acute rheumatic fever (ARF) and rheumatic heart disease are serious autoimmune sequelae to infections with Streptococcus pyogenes. Streptococcal M-proteins have been implicated in ARF pathogenesis. Their interaction with collagen type IV (CIV) is a triggering step that induces generation of collagen-specific auto-antibodies. Electron microscopy of the protein complex between M-protein type 3 (M3-protein) and CIV identified two prominent binding sites of which one is situated in the CB3-region of CIV. In a radioactive binding assay, M3-protein expressing S. pyogenes and S. gordonii bound the CB3-fragment. Detailed analysis of the interactions by surface plasmon resonance measurements and site directed mutagenesis revealed high affinity interactions with dissociation constants in the nanomolar range that depend on the recently described collagen binding motif of streptococcal M-proteins. Because of its role in the induction of disease-related collagen autoimmunity the motif is referred to as "peptide associated with rheumatic fever" (PARF). Both, sera of mice immunized with M3-protein as well as sera from patients with ARF contained anti-CB3 auto-antibodies, indicating their contribution to ARF pathogenesis. The identification of the CB3-region as a binding partner for PARF directs the further approaches to understand the unusual autoimmune pathogenesis of PARF-dependent ARF and forms a molecular basis for a diagnostic test that detects rheumatogenic streptococci.
Conflict of interest statement
Figures


References
-
- WHO. Rheumatic fever and rheumatic heart disease. World Health Organization. 2004:923 923. - PubMed
-
- Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–168. - PubMed
-
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. - PubMed
-
- Dinkla K, Nitsche-Schmitz DP, Barroso V, Reissmann S, Johansson HM, et al. Identification of a streptococcal octapeptide motif involved in acute rheumatic fever. J Biol Chem. 2007;282:18686–18693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

