Transcranial magnetic stimulation, synaptic plasticity and network oscillations
- PMID: 19254380
- PMCID: PMC2653496
- DOI: 10.1186/1743-0003-6-7
Transcranial magnetic stimulation, synaptic plasticity and network oscillations
Abstract
Transcranial magnetic stimulation (TMS) has quickly progressed from a technical curiosity to a bona-fide tool for neurological research. The impetus has been due to the promising results obtained when using TMS to uncover neural processes in normal human subjects, as well as in the treatment of intractable neurological conditions, such as stroke, chronic depression and epilepsy. The basic principle of TMS is that most neuronal axons that fall within the volume of magnetic stimulation become electrically excited, trigger action potentials and release neurotransmitter into the postsynaptic neurons. What happens afterwards remains elusive, especially in the case of repeated stimulation. Here we discuss the likelihood that certain TMS protocols produce long-term changes in cortical synapses akin to long-term potentiation and long-term depression of synaptic transmission. Beyond the synaptic effects, TMS might have consequences on other neuronal processes, such as genetic and protein regulation, and circuit-level patterns, such as network oscillations. Furthermore, TMS might have non-neuronal effects, such as changes in blood flow, which are still poorly understood.
Figures
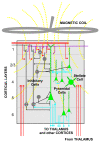

References
-
- Pascual-Leone A, Davey N, Rothwell J, Wassermann EM, Puri BK. Handbook of Transcranial Magnetic Stimulation. London: Hodder Arnold; 2002.
-
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. Cambridge: The MIT Press; 2005.
-
- Wassermann E, Epstein C, Ziemann U. Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press; 2008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

