Fluorescence fluctuation spectroscopy on viral-like particles reveals variable gag stoichiometry
- PMID: 19254556
- PMCID: PMC2717261
- DOI: 10.1016/j.bpj.2008.10.067
Fluorescence fluctuation spectroscopy on viral-like particles reveals variable gag stoichiometry
Abstract
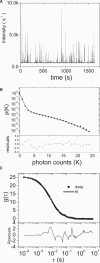
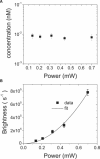
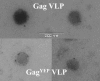
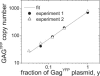
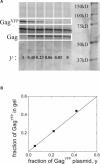
Fluorescence fluctuation spectroscopy determines the brightness, size, and concentration of fluorescent particles from the intensity bursts generated by individual particles passing through a small observation volume. Brightness provides a measure of the number of fluorescently labeled proteins within a complex and has been used previously to determine the stoichiometry of small oligomers in cells. We extend brightness analysis to large macromolecular protein complexes containing thousands of proteins and determine their stoichiometry. This study investigates viral-like particles (VLP) formed from human immunodeficiency virus type 1 (HIV-1) Gag protein expressed in COS-1 cells using fluorescence fluctuation spectroscopy to determine the stoichiometry of HIV-1 Gag within the particles. Control experiments establish that the stoichiometry and size of VLPs are not influenced by labeling of HIV-1 Gag with a fluorescent protein. The experiments further show that the brightness scales linearly with the amount of labeled Gag within the particle. Brightness analysis shows that the Gag stoichiometry of VLPs formed in COS-1 cells is not constant, but varies with the amount of transfected DNA plasmid. We observed HIV-1 Gag stoichiometries ranging from approximately 750 to approximately 2500, whereas the size of the VLPs remains unchanged. This result indicates that large areas of the VLP membrane are void of Gag protein. Therefore, a closed layer of HIV-1 Gag at the membrane is not required for VLP production. This study shows that brightness analysis has the potential to become an important tool for investigating large molecular complexes by providing quantitative information about their size and composition.
Figures

References
-
- Thompson N.L., Lieto A.M., Allen N.W. Recent advances in fluorescence correlation spectroscopy. Curr. Opin. Struct. Biol. 2002;12:634–641. - PubMed
-
- Magde D., Elson E., Webb W.W. Thermodynamic fluctuations in a reacting system: measurement by fluorescence correlation spectroscopy. Phys. Rev. Lett. 1972;29:705–708.
-
- Berland K.M. Fluorescence correlation spectroscopy: a new tool for quantification of molecular interactions. Methods Mol. Biol. 2004;261:383–398. - PubMed
-
- Hwang L.C., Wohland T. Recent advances in fluorescence cross-correlation spectroscopy. Cell Biochem. Biophys. 2007;49:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

