Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression
- PMID: 19254567
- PMCID: PMC2673483
- DOI: 10.1016/j.cmet.2009.01.010
Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression
Abstract
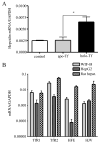
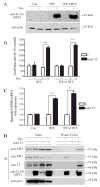
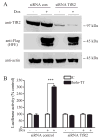
The mechanisms that allow the body to sense iron levels in order to maintain iron homeostasis are unknown. Patients with the most common form of hereditary iron overload have mutations in the hereditary hemochromatosis protein HFE. They have lower levels of hepcidin than unaffected individuals. Hepcidin, a hepatic peptide hormone, negatively regulates iron efflux from the intestines into the blood. We report two hepatic cell lines, WIF-B cells and HepG2 cells transfected with HFE, where hepcidin expression responded to iron-loaded transferrin. The response was abolished when endogenous transferrin receptor 2 (TfR2) was suppressed or in primary hepatocytes lacking either functional TfR2 or HFE. Furthermore, transferrin-treated HepG2 cells transfected with HFE chimeras containing only the alpha3 and cytoplasmic domains could upregulate hepcidin expression. Since the HFE alpha3 domain interacts with TfR2, these results supported our finding that TfR2/HFE complex is required for transcriptional regulation of hepcidin by holo-Tf.
Figures



Comment in
-
Iron sensing as a partnership: HFE and transferrin receptor 2.Cell Metab. 2009 Mar;9(3):211-2. doi: 10.1016/j.cmet.2009.02.004. Cell Metab. 2009. PMID: 19254564
-
Hepcidin regulation by HFE and TFR2: is it enough to give a hepatocyte a complex?Gastroenterology. 2009 Sep;137(3):1173-5; discussion 1175. doi: 10.1053/j.gastro.2009.07.009. Epub 2009 Jul 24. Gastroenterology. 2009. PMID: 19632251 No abstract available.
References
-
- Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, Sly WS, Fleming RE. Decreased liver hepcidin expression in the Hfe knockout mouse. Blood Cells Mol Dis. 2002;29:361–366. - PubMed
-
- Ajioka RS, Kushner JP. Hereditary hemochromatosis. Semin Hematol. 2002;39:235–241. - PubMed
-
- Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403:46–53. - PubMed
-
- Bondi A, Valentino P, Daraio F, Porporato P, Gramaglia E, Carturan S, Gottardi E, Camaschella C, Roetto A. Hepatic expression of hemochromatosis genes in two mouse strains after phlebotomy and iron overload. Haematologica. 2005;90:1161–1167. - PubMed
-
- Bothwell TH, MacPhail AP. Hereditary hemochromatosis: etiologic, pathologic, and clinical aspects. Semin Hematol. 1998;35:55–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

