Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation
- PMID: 19254571
- PMCID: PMC2684673
- DOI: 10.1016/j.cmet.2009.01.012
Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation
Abstract
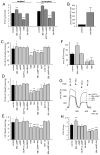
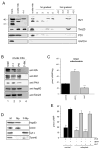
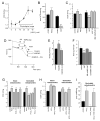
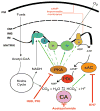
Mitochondria constantly respond to changes in substrate availability and energy utilization to maintain cellular ATP supplies, and at the same time control reactive oxygen radical (ROS) production. Reversible phosphorylation of mitochondrial proteins has been proposed to play a fundamental role in metabolic homeostasis, but very little is known about the signaling pathways involved. We show here that protein kinase A (PKA) regulates ATP production by phosphorylation of mitochondrial proteins, including subunits of cytochrome c oxidase. The cyclic AMP (cAMP), which activates mitochondrial PKA, does not originate from cytoplasmic sources but is generated within mitochondria by the carbon dioxide/bicarbonate-regulated soluble adenylyl cyclase (sAC) in response to metabolically generated carbon dioxide. We demonstrate for the first time the existence of a CO(2)-HCO(3)(-)-sAC-cAMP-PKA (mito-sAC) signaling cascade wholly contained within mitochondria, which serves as a metabolic sensor modulating ATP generation and ROS production in response to nutrient availability.
Figures


References
-
- Akerman KE, Wikstrom MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68:191–197. - PubMed
-
- Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. - PubMed
-
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. - PubMed
-
- Bornfeldt KE. A single second messenger: several possible cellular responses depending on distinct subcellular pools. Circ Res. 2006;99:790–792. - PubMed
-
- Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem. 2002;277:1340–1348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

