Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9
- PMID: 19254573
- PMCID: PMC2673480
- DOI: 10.1016/j.cmet.2009.01.013
Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9
Abstract
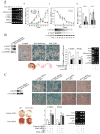
Pref-1 is an EGF repeat-containing transmembrane protein that produces a biologically active soluble form by TACE-mediated cleavage. Although Pref-1 inhibition of adipogenesis has been well established, the specific target(s) of Pref-1 or the Pref-1 function in mesenchymal cell commitment/differentiation are not known. Here, we show that Sox9 downregulation is required for adipocyte differentiation and that Pref-1 inhibits adipocyte differentiation through upregulating Sox9 expression. Sox9 directly binds to the promoter regions of C/EBPbeta and C/EBPdelta to suppress their promoter activity, preventing adipocyte differentiation. Furthermore, we also show that, by inducing Sox9, Pref-1 promotes chondrogenic induction of mesenchymal cells but prevents chondrocyte maturation as well as osteoblast differentiation, with supporting in vivo evidence in Pref-1 null and Pref-1 transgenic mice. Thus, Sox9 is a Pref-1 target, and Pref-1 directs multipotent mesenchymal cells to the chondrogenic lineage but inhibits differentiation into adipocytes as well as osteoblasts and chondrocytes.
Figures









References
-
- Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–852. - PubMed
-
- Bachmann E, Krogh TN, Hojrup P, Skjodt K, Teisner B. Mouse fetal antigen 1 (mFA1), the circulating gene product of mdlk, pref-1 and SCP-1: isolation, characterization and biology. J Reprod Fertil. 1996;107:279–285. - PubMed
-
- da Rocha ST, Ferguson-Smith AC. Genomic imprinting. Curr Biol. 2004;14:R646–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

