Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use
- PMID: 19254592
- PMCID: PMC2853746
- DOI: 10.1016/j.ajog.2008.12.052
Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use
Abstract
Objective: The purpose of this study was to determine changes in bodyweight and composition that result from hormonal contraception.
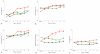
Study design: Dual-energy x-ray absorptiometry was performed at baseline and every 6 months for 3 years for 703 women (African American, 200; white, 247; Hispanic, 256) who were beginning the use of oral contraception (OC; n = 245), depot medroxyprogesterone acetate (DMPA; n = 240), or nonhormonal contraception (NH; n = 218). DMPA discontinuers were observed for up to 2 years to examine the reversibility of the observed changes.
Results: Over 36 months, DMPA users increased their weight (+5.1 kg), body fat (+4.1 kg), percent body fat (+3.4%), and central-to-peripheral fat ratio (+0.1) more than OC and NH users (P < .01). OC use did not cause weight gain. After DMPA discontinuation, NH users lost 0.42 kg in 6 months; OC users gained 0.43 kg in 6 months.
Conclusion: Bodyweight and fat significantly increase with the use of DMPA. After discontinuation of DMPA, some decrease in bodyweight and fat occurs when NH is used.
Figures

References
-
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25–35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006;74:90–9. - PubMed
-
- Polaneczky M, Liblanc M. Long-term depot medroxyprogesterone acetate (Depo-Provera) use in inner-city adolescents. J Adolesc Health. 1998;23:81–8. - PubMed
-
- Templeman C, Boyd H, Hertweck SP. Depomedroxyprogesterone acetate use and weight gain among adolescents. J Pediatr Adolesc Gynecol. 2000;13:45–6. - PubMed
-
- Bahamondes L, Del Castillo S, Tabares G, Arce XE, Perrotti M, Petta C. Comparison of weight increase in users of depot medroxyprogesterone acetate and copper IUD up to 5 years. Contraception. 2001;64:223–5. - PubMed
-
- Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot-medroxyprogesterone acetate for contraception. Int J Obes (Lond) 2005;29:1252–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

