Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice
- PMID: 19254681
- PMCID: PMC2695717
- DOI: 10.1053/j.gastro.2008.12.051
Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice
Abstract
Background & aims: Vitamin D receptor (VDR)-knockout mice develop severe hypocalcemia and rickets, accompanied by disruption of active intestinal calcium absorption. To specifically study the effects of VDR in intestinal calcium absorption, we investigated whether restoration of intestinal VDR is sufficient to recover the phenotype of VDR-knockout mice.
Methods: We generated mice with intestine-specific transgenic expression of human VDR and crossed them to VDR knockout mice. The intestine, kidney, and bone phenotypes of the VDR- knockout mice with intestine-specific expression of human VDR (knockout/transgenic [KO/TG]) were analyzed.
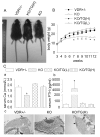
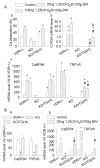
Results: Transgenic expression of VDR in the intestine of VDR-knockout mice normalized duodenal vitamin D-regulated calcium absorption as well as vitamin D-regulated calcium binding protein D9k and TRPV6 gene expression in the duodenum and proximal colon. As a result, animal growth and the serum levels of calcium and parathyroid hormone were normalized in KO/TG mice. Other phenotypes were revealed when calcium metabolism was normalized in KO/TG mice: serum 1,25 dihydroxyvitamin D levels were higher in KO/TG mice than normal mice owing to reduced renal expression of the vitamin D-degrading enzyme CYP24, urinary calcium excretion was higher and associated with lower renal calcium binding protein D9k and calcium binding protein D28k than normal mice, and bone density and volume increased in KO/TG compared with normal mice owing to increased mineral apposition rate and osteoblast number.
Conclusions: Intestinal VDR and vitamin D-regulated intestinal calcium absorption are critical for controlling whole-body calcium metabolism in growing mice. Normalizing intestinal calcium absorption and metabolism reveals essential roles for VDR in control of bone formation and renal control of serum 1,25(OH)2D and urinary calcium excretion.
Conflict of interest statement
Conflicts of interest: There are no conflicts of interest to disclose.
Figures


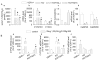
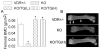
References
-
- Fleet JC. Molecular Regulation of Calcium Metabolism. In: Weaver CM, Heaney RP, editors. Calcium in Human Health. Totowa, NJ: Humana Press; 2006. pp. 163–190.
-
- Wood RJ, Fleet JC, Cashman K, et al. Intestinal calcium absorption in the aged rat: evidence of intestinal resistance to 1,25(OH)2 vitamin D. Endocrinology. 1998;139:3843–3848. - PubMed
-
- Song Y, Peng X, Porta A, et al. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. - PubMed
-
- Meyer MB, Watanuki M, Kim S, et al. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–1461. - PubMed
-
- Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250:G561–G569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

