Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice
- PMID: 19254849
- PMCID: PMC2745339
- DOI: 10.1016/j.cyto.2008.12.019
Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice
Abstract
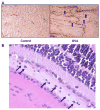
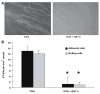
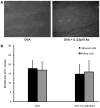
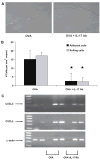
T cell-mediated uveitis is strongly associated with many systemic inflammatory disorders. Th17 cells are a novel T cell subset characterized by production of interleukin (IL)-17. In this study, we used DO11.10 mice to investigate the role of IL-17 in the pathogenesis of uveitis. CD4(+) T cells in DO11.10 mice are genetically engineered to react with ovalbumin (OVA). IL-17 expression was determined by real-time PCR and ELISPOT. Uveitis was induced by intravitreal injection of OVA, and ocular inflammation was evaluated by intravital microscopy. OVA challenge significantly induced IL-17 production by DO11.10 splenocytes in vitro. Next, we examined whether OVA challenge could elicit local inflammation and induce IL-17 in vivo. OVA elicited marked neutrophil-predominant inflammatory cell infiltration in the eyes. This leukocyte influx was mediated by CD4(+) lymphocytes as evidenced by significant inhibition of the ocular inflammation by CD4+ depleting antibody. Compared to control mice, OVA treatment induced IL-17 expression. Moreover, anti-IL-17 antibody markedly reduced OVA-mediated ocular inflammation. Finally, the neutralization of IL-17 attenuated ocular expression of CXCL2 and CXCL5, two cytokines which are chemotactic for neutrophils. Our study suggests that IL-17 is implicated in the pathogenesis of this T cell-mediated model of uveitis in part through neutrophil chemotaxis as a downstream effect of IL-17.
Figures








References
-
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. discussion 500. - PubMed
-
- Nussenblatt RB. The natural history of uveitis. International Ophthalmology. 1990;14(5–6):303–8. - PubMed
-
- Becker MD, Adamus G, Davey MP, Rosenbaum JT. The role of T cells in autoimmune uveitis. Ocular Immunology & Inflammation. 2000;8(2):93–100. - PubMed
-
- Martin TM, Smith JR, Rosenbaum JT. Anterior uveitis: current concepts of pathogenesis and interactions with the spondyloarthropathies. Current Opinion in Rheumatology. 2002;14(4):337–41. - PubMed
-
- Atalla L, Linker-Israeli M, Steinman L, Rao NA. Inhibition of autoimmune uveitis by anti-CD4 antibody. Investigative Ophthalmology & Visual Science. 1990;31(7):1264–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

