Determining the helical tilt of membrane peptides using electron paramagnetic resonance spectroscopy
- PMID: 19254856
- PMCID: PMC2666113
- DOI: 10.1016/j.jmr.2008.12.007
Determining the helical tilt of membrane peptides using electron paramagnetic resonance spectroscopy
Abstract
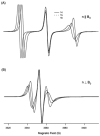
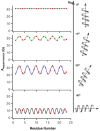
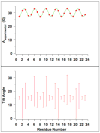
Theoretical calculations of hyperfine splitting values derived from the EPR spectra of TOAC spin-labeled rigid aligned alpha-helical membrane peptides reveal a unique periodic variation. In the absence of helical motion, a plot of the corresponding hyperfine splitting values as a function of residue number results in a sinusoidal curve that depends on the helical tilt angle that the peptide makes with respect to the magnetic field. Motion about the long helical axis reduces the amplitude of the curve and averages out the corresponding hyperfine splitting values. The corresponding spectra can be used to determine the director axis tilt angle from the TOAC spin label, which can be used to calculate the helical tilt angle due to the rigidity of the TOAC spin label. Additionally, this paper describes a method to experimentally determine this helical tilt angle from the hyperfine splitting values of three consecutive residues.
Figures



References
-
- Arkin IT, MacKenzie KR, Fisher L, Aimoto S, Engelman DM, Smith SO. Mapping the lipid-exposed surfaces of membrane proteins. Nat Struct Biol. 1996;3:240–243. - PubMed
-
- Nagy JK, Lau FW, Bowie JU, Sanders CR. Mapping the oligomeric interface of diacylglycerol kinase by engineered thiol cross-linking: Homologous sites in the transmembrane domain. Biochemistry. 2000;39:4154–4164. - PubMed
-
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo AL, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
-
- Fu DX, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

