Nicastrin is dispensable for gamma-secretase protease activity in the presence of specific presenilin mutations
- PMID: 19254953
- PMCID: PMC2676034
- DOI: 10.1074/jbc.M807653200
Nicastrin is dispensable for gamma-secretase protease activity in the presence of specific presenilin mutations
Abstract
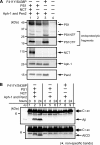
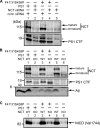
gamma-Secretase is a multisubunit membrane protein complex consisting of presenilin (PS1), nicastrin (NCT), anterior pharynx-1, and presenilin enhancer 2. To analyze the activity of familial Alzheimer disease mutants and to understand the roles of the subunits, we established a yeast transcriptional activator Gal4p system with artificial gamma-secretase substrates containing amyloid precursor protein or Notch fragments. The gamma-secretase activities were evaluated by transcriptional activation of reporter genes upon Gal4p release from the membrane-bound substrates, i.e. growth of yeast on histidine and adenine, or beta-galactosidase assay. We screened and evaluated gamma-secretase mutants using this reconstitution system in yeast, which does not possess endogenous gamma-secretase activity. When we introduced familial Alzheimer mutants of PS1 in this system, their activities were shown to be loss of function. Although the protease activity of wild type PS1 depends on the other three subunits introduced, we obtained 15 new PS1 mutants, which are active in the absence of NCT. They possessed a S438P mutation at the ninth transmembrane domain (TM9) together with one missense mutation distributed through transmembrane and loop regions. These mutations were not related to familial Alzheimer mutations of PS1 as identified so far. The S438P mutant was partially active but required other mutations for full activation. Results of the beta-galactosidase assay suggested that they have wild type protease activities, which were further confirmed by the endoproteolysis of PS1, amyloid beta peptides, and Notch intracellular domain production in mammalian cells. These results suggest that NCT is dispensable for the protease activity of gamma-secretase.
Figures

References
-
- Selkoe, D. J. (2001) Physiol. Rev. 81 741-766 - PubMed
-
- Edbauer, D., Winkler, E., Regula, J. T., Pesold, B., Steiner, H., and Haass, C. (2003) Nat. Cell Biol. 5 486-488 - PubMed
-
- Laudon, H., Hansson, E. M., Melén, K., Bergman, A., Farmery, M. R., Winblad, B., Lendahl, U., von Heijne, G., and Näslund, J. (2005) J. Biol. Chem. 280 35352-35360 - PubMed
-
- Oh, Y. S., and Turner, R. J. (2005) Biochemistry 44 11821-11828 - PubMed
-
- Spasic, D., Tolia, A., Dillen, K., Baert, V., De Strooper, B., Vrijens, S., and Annaert, W. (2006) J. Biol. Chem. 281 26569-26577 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

