Impact of disclosure of HIV infection on health-related quality of life among children and adolescents with HIV infection
- PMID: 19255023
- PMCID: PMC2697844
- DOI: 10.1542/peds.2008-1290
Impact of disclosure of HIV infection on health-related quality of life among children and adolescents with HIV infection
Abstract
Background: Little is known concerning the impact of HIV status disclosure on quality of life, leaving clinicians and families to rely on research of children with other terminal illnesses.
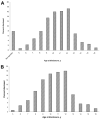
Objectives: The purpose of this work was to examine the impact of HIV disclosure on pediatric quality of life and to describe the distribution of age at disclosure in a perinatally infected pediatric population. METHODS. A longitudinal analysis was conducted of perinatally HIV-infected youth >/=5 years of age enrolled in a prospective cohort study, Pediatric AIDS Clinical Trials Group 219C, with >/=1 study visit before and after HIV disclosure. Age-specific quality-of-life instruments were completed by primary caregivers at routine study visits. The distribution of age at disclosure was summarized. Six quality-of-life domains were assessed, including general health perception, symptom distress, psychological status, health care utilization, physical functioning, and social/role functioning. For each domain, mixed-effects models were fit to estimate the effect of disclosure on quality of life.
Results: A total of 395 children with 2423 study visits were analyzed (1317 predisclosure visits and 1106 postdisclosure visits). The median age at disclosure was estimated to be 11 years. Older age at disclosure was associated with earlier year of birth. Mean domain scores were not significantly different at the last undisclosed visit compared with the first disclosed visit, with the exception of general health perception. When all of the visits were considered, 5 of 6 mean domain scores were lower after disclosure, although the differences were not significant. In mixed-effects models, disclosure did not significantly impact quality of life for any domain.
Conclusions: Age at disclosure decreased significantly over time. There were no statistically significant differences between predisclosure and postdisclosure quality of life; therefore, disclosure should be encouraged at an appropriate time.
Figures


References
-
- Berk DR, Falkovitz-Halpern MS, Hill DW, et al. Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. JAMA. 2005;293(18):2221–2231. - PubMed
-
- Wiener L, Lyon M. HIV disclosure: who knows? who needs to know?: clinical and ethical considerations. In: Lyon M, D'Angelo L, editors. Teenagers, HIV, and AIDS: Insights From Youths Living With the Virus. Westport, CT: Praeger Publishers; 2006. pp. 105–126.
-
- Patel K, Hernan MA, Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46(4):507–515. - PubMed
-
- de Martino M, Tovo PA, Balducci M, et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection: Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA. 2000;284(2):190–197. - PubMed
-
- Lester P, Chesney M, Cooke M, et al. When the time comes to talk about HIV: factors associated with diagnostic disclosure and emotional distress in HIV-infected children. J Acquir Immune Defic Syndr. 2002;31(3):309–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

