Predominant modifier of extreme liver cancer susceptibility in C57BR/cdJ female mice localized to 6 Mb on chromosome 17
- PMID: 19255062
- PMCID: PMC2675651
- DOI: 10.1093/carcin/bgp054
Predominant modifier of extreme liver cancer susceptibility in C57BR/cdJ female mice localized to 6 Mb on chromosome 17
Abstract
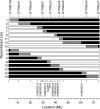
Sex hormones influence the susceptibility of inbred mice to liver cancer. C57BR/cdJ (BR) females are extremely susceptible to spontaneous and chemically induced liver tumors, in part due to a lack of protection against hepatocarcinogenesis normally offered by ovarian hormones. BR males are also moderately susceptible, and the susceptibility of both sexes of BR mice to liver tumors induced with N,N-diethylnitrosamine relative to the resistant C57BL/6J (B6) strain is caused by two loci designated Hcf1 and Hcf2 (hepatocarcinogenesis in females) located on chromosomes 17 and 1, respectively. The Hcf1 locus on chromosome 17 is the predominant modifier of liver cancer in BR mice. To validate the existence of this locus and investigate its potential interaction with Hcf2, congenic mice for each region were generated. Homozygosity for the B6.BR(D17Mit164-D17Mit2) region resulted in a 4-fold increase in liver tumor multiplicity in females and a 4.5-fold increase in males compared with B6 controls. A series of 16 recombinants covering the entire congenic region was developed to further narrow the area containing Hcf1. Susceptible heterozygous recombinants demonstrated a 3- to 7-fold effect in females and a 1.5- to 2-fold effect in males compared with B6 siblings. The effect in susceptible lines completely recapitulated the susceptibility of heterozygous full-length chromosome 17 congenics and furthermore narrowed the location of the Hcf1 locus to a single region of the chromosome from 30.05 to 35.83 Mb.
Figures

References
-
- Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. - PubMed
-
- Tanaka K, et al. Serum testosterone: estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res. 2000;60:5106–5110. - PubMed
-
- Yu MW, et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J. Natl Cancer Inst. 2001;93:1644–1651. - PubMed
-
- Moradpour D, et al. Pathogenesis of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2005;17:477–483. - PubMed
-
- Smith GS, et al. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F1 hybrids. J. Natl Cancer Inst. 1973;50:1195–1213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

