Structural basis for the sequence-dependent effects of platinum-DNA adducts
- PMID: 19255091
- PMCID: PMC2677858
- DOI: 10.1093/nar/gkp029
Structural basis for the sequence-dependent effects of platinum-DNA adducts
Abstract
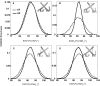
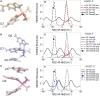
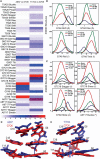
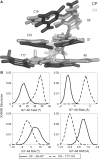
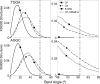
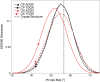
The differences in efficacy and molecular mechanisms of platinum based anti-cancer drugs cisplatin (CP) and oxaliplatin (OX) have been hypothesized to be in part due to the differential binding affinity of cellular and damage recognition proteins to CP and OX adducts formed on adjacent guanines in genomic DNA. HMGB1a in particular exhibits higher binding affinity to CP-GG adducts, and the extent of discrimination between CP- and OX-GG adducts is dependent on the bases flanking the adducts. However, the structural basis for this differential binding is not known. Here, we show that the conformational dynamics of CP- and OX-GG adducts are distinct and depend on the sequence context of the adduct. Molecular dynamics simulations of the Pt-GG adducts in the TGGA sequence context revealed that even though the major conformations of CP- and OX-GG adducts were similar, the minor conformations were distinct. Using the pattern of hydrogen bond formation between the Pt-ammines and the adjacent DNA bases, we identified the major and minor conformations sampled by Pt-DNA. We found that the minor conformations sampled exclusively by the CP-GG adduct exhibit structural properties that favor binding by HMGB1a, which may explain its higher binding affinity to CP-GG adducts, while these conformations are not sampled by OX-GG adducts because of the constraints imposed by its cyclohexane ring, which may explain the negligible binding affinity of HMGB1a for OX-GG adducts in the TGGA sequence context. Based on these results, we postulate that the constraints imposed by the cyclohexane ring of OX affect the DNA conformations explored by OX-GG adduct compared to those of CP-GG adduct, which may influence the binding affinities of HMG-domain proteins for Pt-GG adducts, and that these conformations are further influenced by the DNA sequence context of the Pt-GG adduct.
Figures
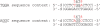


References
-
- Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit. Rev. Oncol. Hematol. 2005;53:L3–L11. - PubMed
-
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999;99:2467–2498. - PubMed
-
- Page JD, Husain I, Sancar A, Chaney SG. Effect of the diaminocyclohexane carrier ligand on platinum adduct formation, repair, and lethality. Biochemistry. 1990;29:1016–1024. - PubMed
-
- Jennerwein MM, Eastman A, Khokhar AR. Characterization of adducts produced in DNA by isomeric 1,2-diaminocyclohexaneplatinum(II) complexes. Chem. Biol. Interact. 1989;70:39–49. - PubMed
-
- Woynarowski JM, Chapman WG, Napier C, Herzig MCS, Juniewicz P. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol. Pharmacol. 1998;54:770–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

