Acid-sensing ion channels in neurones of the rat suprachiasmatic nucleus
- PMID: 19255120
- PMCID: PMC2683960
- DOI: 10.1113/jphysiol.2008.166918
Acid-sensing ion channels in neurones of the rat suprachiasmatic nucleus
Abstract
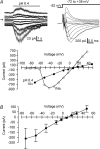
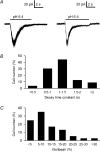
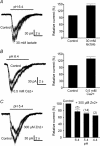
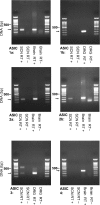
We used reduced slice reparations to study ASIC-like currents in the rat central clock suprachiasmatic nucleus (SCN). In reduced SCN preparations, a drop of extracellular pH evoked a desensitizing inward current to excite SCN neurones to fire at higher rates. Under voltage-clamped conditions, all SCN neurones responded to a 5 s pH step to 6.4 with an inward current that decayed with an average time constant of 1.2 s to 10% of the peak at the end of step. The current was blocked by amiloride with an IC(50) of 14 microm and was carried mainly by Na(+), suggesting an origin of ASIC-like channels. The SCN neurones were sensitive to neutral pH, with 94% of cells responding to pH 7.0 with an inward current. The study of sensitivity to pH between 7.0 and 4.4 revealed a two-component dose-dependent H(+) activation in most SCN neurones, with the first component (85% in amplitude) having a pH(50) of 6.6, and the second (15%) a pH(50) of 5. The ASIC-like currents were potentiated by lactate and low Ca(2+), but were inhibited by Zn(2+). RT-PCR analysis demonstrated the presence of mRNA for ASIC1a, 2a, 2b, and 3 in SCN. Compared to other central neurones, the unique presence of ASIC3 along with ASIC1a in SCN neurones may contribute to the high pH sensitivity and unusual inhibition by Zn(2+). The high pH sensitivity suggests that the SCN neurones are susceptive to extracellular acidification of physiological origins and that the ASIC current might play a role in regulating SCN excitability.
Figures



Similar articles
-
Functional characterization of acid-sensing ion channels in cultured neurons of rat inferior colliculus.Neuroscience. 2008 Jun 23;154(2):461-72. doi: 10.1016/j.neuroscience.2008.03.040. Epub 2008 Mar 26. Neuroscience. 2008. PMID: 18456416
-
Properties of the proton-evoked currents and their modulation by Ca2+ and Zn2+ in the acutely dissociated hippocampus CA1 neurons.Brain Res. 2004 Aug 13;1017(1-2):197-207. doi: 10.1016/j.brainres.2004.05.046. Brain Res. 2004. PMID: 15261115
-
ASIC-like, proton-activated currents in rat hippocampal neurons.J Physiol. 2002 Mar 1;539(Pt 2):485-94. doi: 10.1113/jphysiol.2001.014837. J Physiol. 2002. PMID: 11882680 Free PMC article.
-
Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases.Curr Opin Pharmacol. 2008 Feb;8(1):25-32. doi: 10.1016/j.coph.2007.09.001. Epub 2007 Oct 22. Curr Opin Pharmacol. 2008. PMID: 17945532 Free PMC article. Review.
-
Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract.Am J Physiol Gastrointest Liver Physiol. 2007 Mar;292(3):G699-705. doi: 10.1152/ajpgi.00517.2006. Epub 2006 Nov 22. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17122365 Free PMC article. Review.
Cited by
-
Role of Na⁺/Ca²⁺ exchanger in Ca²⁺ homeostasis in rat suprachiasmatic nucleus neurons.J Neurophysiol. 2015 Apr 1;113(7):2114-26. doi: 10.1152/jn.00404.2014. Epub 2015 Jan 7. J Neurophysiol. 2015. PMID: 25568156 Free PMC article.
-
ASICs as therapeutic targets for migraine.Neuropharmacology. 2015 Jul;94:64-71. doi: 10.1016/j.neuropharm.2014.12.015. Epub 2015 Jan 9. Neuropharmacology. 2015. PMID: 25582295 Free PMC article. Review.
-
ASIC3 channels in multimodal sensory perception.ACS Chem Neurosci. 2011 Jan 19;2(1):26-37. doi: 10.1021/cn100094b. Epub 2010 Nov 12. ACS Chem Neurosci. 2011. PMID: 22778854 Free PMC article. Review.
-
Expression of ASIC3 in the Trigeminal Nucleus Caudalis Plays a Role in a Rat Model of Recurrent Migraine.J Mol Neurosci. 2018 Sep;66(1):44-52. doi: 10.1007/s12031-018-1113-3. Epub 2018 Sep 12. J Mol Neurosci. 2018. PMID: 30209688
-
Hydrogen Sulfide Upregulates Acid-sensing Ion Channels via the MAPK-Erk1/2 Signaling Pathway.Function (Oxf). 2021 Feb 19;2(2):zqab007. doi: 10.1093/function/zqab007. eCollection 2021. Function (Oxf). 2021. PMID: 35330812 Free PMC article.
References
-
- Akopian AN, Chen C-C, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. - PubMed
-
- Alberti KG, Cuthbert C. The hydrogen ion in normal metabolism: a review. Ciba Found Symp. 1982;87:1–19. - PubMed
-
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. - PubMed
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Babini E, Paukert M, Geisler HS, Gründer S. Alternative splicing and interaction with di-and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

