Nuclear ferritin: a ferritoid-ferritin complex in corneal epithelial cells
- PMID: 19255152
- PMCID: PMC4793724
- DOI: 10.1167/iovs.08-3170
Nuclear ferritin: a ferritoid-ferritin complex in corneal epithelial cells
Abstract
Purpose: Ferritin is an iron storage protein that is generally cytoplasmic. However, in embryonic avian corneal epithelial (CE) cells, the authors previously observed that the ferritin was predominantly nuclear. They also obtained evidence that this ferritin protects DNA from oxidative damage by UV light and hydrogen peroxide and that the nuclear localization involves a tissue-specific nuclear transporter, termed ferritoid. In the present investigation, the authors have determined additional properties of the nuclear ferritoid-ferritin complexes.
Methods: For biochemical characterization, a combination of molecular sieve chromatography, immunoblotting, and nuclear-cytoplasmic fractionation was used; DNA binding was analyzed by electrophoretic mobility shift assay.
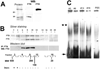
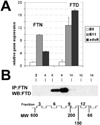
Results: The CE nuclear ferritin complex has characteristics that differentiate it from a "typical" cytoplasmic ferritin, including the presence of ferritin and ferritoid subunits; a molecular weight of approximately 260 kDa, which is approximately half that of cytoplasmic ferritin; its iron content, which is below our limits of detection; and its ability to bind to DNA.
Conclusions: Within CE cell nuclei, ferritin and ferritoid are coassembled into stable complex(es) present in embryonic and adult corneas. Thus, ferritoid not only serves transiently as a nuclear transporter for ferritin, it remains as a component of a unique ferritoid-ferritin nuclear complex.
Figures


Similar articles
-
Developmental regulation of the nuclear ferritoid-ferritin complex of avian corneal epithelial cells: roles of systemic factors and thyroxine.Exp Eye Res. 2009 Dec;89(6):854-62. doi: 10.1016/j.exer.2009.07.007. Epub 2009 Jul 21. Exp Eye Res. 2009. PMID: 19627987 Free PMC article.
-
Ferritoid, a tissue-specific nuclear transport protein for ferritin in corneal epithelial cells.J Biol Chem. 2003 Jun 27;278(26):23963-70. doi: 10.1074/jbc.M210050200. Epub 2003 Apr 15. J Biol Chem. 2003. PMID: 12697769
-
Corneal epithelial nuclear ferritin: developmental regulation of ferritin and its nuclear transporter ferritoid.Dev Dyn. 2008 Sep;237(9):2529-41. doi: 10.1002/dvdy.21691. Dev Dyn. 2008. PMID: 18729209 Free PMC article.
-
Nuclear ferritin in corneal epithelial cells: tissue-specific nuclear transport and protection from UV-damage.Prog Retin Eye Res. 2005 Mar;24(2):139-59. doi: 10.1016/j.preteyeres.2004.08.004. Epub 2004 Nov 11. Prog Retin Eye Res. 2005. PMID: 15610971 Review.
-
Nuclear ferritin: A new role for ferritin in cell biology.Biochim Biophys Acta. 2010 Aug;1800(8):793-7. doi: 10.1016/j.bbagen.2010.03.017. Epub 2010 Mar 25. Biochim Biophys Acta. 2010. PMID: 20347012 Review.
Cited by
-
Phosphorylation regulates the ferritoid-ferritin interaction and nuclear transport.J Cell Biochem. 2009 Jun 1;107(3):528-36. doi: 10.1002/jcb.22154. J Cell Biochem. 2009. PMID: 19360808 Free PMC article.
-
Intraocular Oxygen and Antioxidant Status: New Insights on the Effect of Vitrectomy and Glaucoma Pathogenesis.Am J Ophthalmol. 2019 Jul;203:12-25. doi: 10.1016/j.ajo.2019.02.008. Epub 2019 Feb 15. Am J Ophthalmol. 2019. PMID: 30772349 Free PMC article.
-
Nuclear ferritin mediated regulation of JNK signaling in corneal epithelial cells.Exp Eye Res. 2016 Apr;145:337-340. doi: 10.1016/j.exer.2016.02.002. Epub 2016 Feb 13. Exp Eye Res. 2016. PMID: 26880020 Free PMC article.
-
Biomedical Applications of Lactoferrin on the Ocular Surface.Pharmaceutics. 2023 Mar 7;15(3):865. doi: 10.3390/pharmaceutics15030865. Pharmaceutics. 2023. PMID: 36986726 Free PMC article. Review.
-
Developmental regulation of the nuclear ferritoid-ferritin complex of avian corneal epithelial cells: roles of systemic factors and thyroxine.Exp Eye Res. 2009 Dec;89(6):854-62. doi: 10.1016/j.exer.2009.07.007. Epub 2009 Jul 21. Exp Eye Res. 2009. PMID: 19627987 Free PMC article.
References
-
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med. 1995;18:321–336. - PubMed
-
- Henle ES, Luo Y, Gassmann W, Linn S. Oxidative damage to DNA constituents by iron-mediated Fenton reactions: the deoxyguanosine family. J Biol Chem. 1996;271:21177–21186. - PubMed
-
- Luo Y, Henle ES, Linn S. Oxidative damage to DNA constituents by iron-mediated Fenton reactions: the deoxycytidine family. J Biol Chem. 1996;271:21167–21176. - PubMed
-
- Shimmura S, Suematsu M, Shimoyama M, Tsubota K, Oguchi Y, Ishimura Y. Subthreshold UV radiation-induced peroxide formation in cultured corneal epithelial cells: the protective effects of lactoferrin. Exp Eye Res. 1996;63:519–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

