Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria
- PMID: 19255159
- PMCID: PMC3001322
- DOI: 10.1167/iovs.08-3240
Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria
Abstract
Purpose: To analyze the relationship between ARMS2 and HTRA1 in the association with age-related macular degeneration (AMD) in an independent case-control dataset and to investigate the subcellular localization of the ARMS2 protein in an in vitro system.
Methods: Two SNPs in ARMS2 and HTRA1 were genotyped in 685 cases and 269 controls by a genotyping assay. Allelic association was tested by a chi(2) test. A likelihood ratio test (LRT) of full versus reduced models was used to analyze the interaction between ARMS2 and smoking and HTRA1 and smoking, after adjustment for CFH and age. Immunofluorescence and immunoblot were applied to localize ARMS2 in retinal epithelial ARPE-19 cells and COS7 cell transfected by ARMS2 constructs.
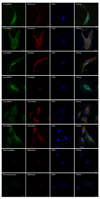
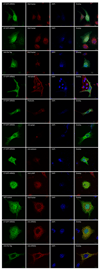
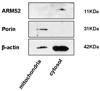
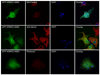
Results: Both significantly associated SNP rs10490924 and rs11200638 (P < 0.0001) are in strong linkage disequilibrium (LD; D' = 0.97, r(2) = 0.93) that generates virtually identical association test and odds ratios. In separate logistic regression models, the interaction effect for both smoking with ARMS2 and with HTRA1 was not statistically significant. Immunofluorescence and immunoblot show that both endogenous and exogenous ARMS2 are mainly distributed in the cytosol, not the mitochondria. Compared with the wild-type, ARMS2 A69S is more likely to be associated with the cytoskeleton in COS7 cells.
Conclusions: The significant associations in ARMS2 and HTRA1 are with polymorphisms in strong LD that confer virtually identical risks, preventing differentiation at the statistical level. ARMS2 was mainly distributed in the cytosol, not in the mitochondrial outer membrane as previously reported, suggesting that ARMS2 may not confer risk to AMD through the mitochondrial pathway.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. - PubMed
-
- Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

