Identification of progenitor cells that contribute to heterotopic skeletogenesis
- PMID: 19255227
- PMCID: PMC2663346
- DOI: 10.2106/JBJS.H.01177
Identification of progenitor cells that contribute to heterotopic skeletogenesis
Abstract
Background: Individuals who have fibrodysplasia ossificans progressiva develop an ectopic skeleton because of genetic dysregulation of bone morphogenetic protein (BMP) signaling in the presence of inflammatory triggers. The identity of progenitor cells that contribute to various stages of BMP-induced heterotopic ossification relevant to fibrodysplasia ossificans progressiva and related disorders is unknown. An understanding of the cellular basis of heterotopic ossification will aid in the development of targeted, cell-specific therapies for the treatment and prevention of heterotopic ossification.
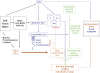
Methods: We used Cre/loxP lineage tracing methods in the mouse to identify cell lineages that contribute to all stages of heterotopic ossification. Specific cell populations were permanently labeled by crossing lineage-specific Cre mice with the Cre-dependent reporter mice R26R and R26R-EYFP. Two mouse models were used to induce heterotopic ossification: (1) intramuscular injection of BMP2/Matrigel and (2) cardiotoxin-induced skeletal muscle injury in transgenic mice that misexpress BMP4 at the neuromuscular junction. The contribution of labeled cells to fibroproliferative lesions, cartilage, and bone was evaluated histologically by light and fluorescence microscopy. The cell types evaluated as possible progenitors included skeletal muscle stem cells (MyoD-Cre), endothelium and endothelial precursors (Tie2-Cre), and vascular smooth muscle (Smooth Muscle Myosin Heavy Chain-Cre [SMMHC-Cre]).
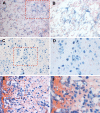
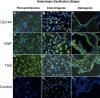
Results: Vascular smooth muscle cells did not contribute to any stage of heterotopic ossification in either mouse model. Despite the osteogenic response of cultured skeletal myoblasts to BMPs, skeletal muscle precursors in vivo contributed minimally to heterotopic ossification (<5%), and this contribution was not increased by cardiotoxin injection, which induces muscle regeneration and mobilizes muscle stem cells. In contrast, cells that expressed the vascular endothelial marker Tie2/Tek at some time in their developmental history contributed robustly to the fibroproliferative, chondrogenic, and osteogenic stages of the evolving heterotopic endochondral anlagen. Importantly, endothelial markers were expressed by cells at all stages of heterotopic ossification. Finally, muscle injury and associated inflammation were sufficient to trigger fibrodysplasia ossificans progressiva-like heterotopic ossification in a setting of chronically stimulated BMP activity.
Conclusions: Tie2-expressing progenitor cells, which are endothelial precursors, respond to an inflammatory trigger, differentiate through an endochondral pathway, contribute to every stage of the heterotopic endochondral anlagen, and form heterotopic bone in response to overactive BMP signaling in animal models of fibrodysplasia ossificans progressiva. Thus, the ectopic skeleton is not only supplied by a rich vasculature, but appears to be constructed in part by cells of vascular origin. Further, these data strongly suggest that dysregulation of the BMP signaling pathway and an inflammatory microenvironment are both required for the formation of fibrodysplasia ossificans progressiva-like lesions.
Figures
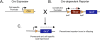


References
-
- Kaplan FS, Groppe J, Pignolo RJ, Shore EM. Morphogen receptor genes and metamorphogenes: skeleton keys to metamorphosis. Ann N Y Acad Sci. 2007;1116:113-33. - PubMed
-
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970-82. - PubMed
-
- Shore EM, Xu M, Feldman JG, Fenstermacher DA, Choi TJ, Choi IH, Connor JM, Delai P, Glasser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525-7. - PubMed
-
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528-34. - PubMed
-
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

