Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming
- PMID: 19255244
- PMCID: PMC2686405
- DOI: 10.1083/jcb.200812026
Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming
Abstract
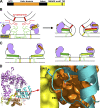
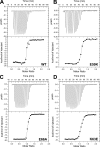
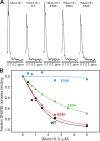
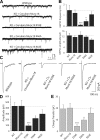
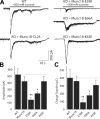
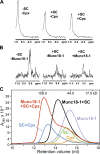
Munc18-1 and soluble NSF attachment protein receptors (SNAREs) are critical for synaptic vesicle fusion. Munc18-1 binds to the SNARE syntaxin-1 folded into a closed conformation and to SNARE complexes containing open syntaxin-1. Understanding which steps in fusion depend on the latter interaction and whether Munc18-1 competes with other factors such as complexins for SNARE complex binding is critical to elucidate the mechanisms involved. In this study, we show that lentiviral expression of Munc18-1 rescues abrogation of release in Munc18-1 knockout mice. We describe point mutations in Munc18-1 that preserve tight binding to closed syntaxin-1 but markedly disrupt Munc18-1 binding to SNARE complexes containing open syntaxin-1. Lentiviral rescue experiments reveal that such disruption selectively impairs synaptic vesicle priming but not Ca(2+)-triggered fusion of primed vesicles. We also find that Munc18-1 and complexin-1 bind simultaneously to SNARE complexes. These results suggest that Munc18-1 binding to SNARE complexes mediates synaptic vesicle priming and that the resulting primed state involves a Munc18-1-SNARE-complexin macromolecular assembly that is poised for Ca(2+) triggering of fusion.
Figures

References
-
- Arac D., Murphy T., Rizo J. 2003. Facile detection of protein-protein interactions by one-dimensional NMR spectroscopy.Biochemistry. 42:2774–2780 - PubMed
-
- Atwell S., Ultsch M., De Vos A.M., Wells J.A. 1997. Structural plasticity in a remodeled protein-protein interface.Science. 278:1125–1128 - PubMed
-
- Brunger A.T. 2005. Structure and function of SNARE and SNARE-interacting proteins.Q. Rev. Biophys. 38:1–47 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

