Mammalian end binding proteins control persistent microtubule growth
- PMID: 19255245
- PMCID: PMC2686402
- DOI: 10.1083/jcb.200807179
Mammalian end binding proteins control persistent microtubule growth
Abstract
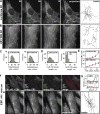
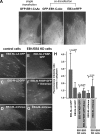
End binding proteins (EBs) are highly conserved core components of microtubule plus-end tracking protein networks. Here we investigated the roles of the three mammalian EBs in controlling microtubule dynamics and analyzed the domains involved. Protein depletion and rescue experiments showed that EB1 and EB3, but not EB2, promote persistent microtubule growth by suppressing catastrophes. Furthermore, we demonstrated in vitro and in cells that the EB plus-end tracking behavior depends on the calponin homology domain but does not require dimer formation. In contrast, dimerization is necessary for the EB anti-catastrophe activity in cells; this explains why the EB1 dimerization domain, which disrupts native EB dimers, exhibits a dominant-negative effect. When microtubule dynamics is reconstituted with purified tubulin, EBs promote rather than inhibit catastrophes, suggesting that in cells EBs prevent catastrophes by counteracting other microtubule regulators. This probably occurs through their action on microtubule ends, because catastrophe suppression does not require the EB domains needed for binding to known EB partners.
Figures






References
-
- Akhmanova A., Steinmetz M.O. 2008. Tracking the ends: a dynamic protein network controls the fate of microtubule tips.Nat. Rev. Mol. Cell Biol. 9:309–322 - PubMed
-
- Arnold K., Bordoli L., Kopp J., Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling.Bioinformatics. 22:195–201 - PubMed
-
- Bieling P., Laan L., Schek H., Munteanu E.L., Sandblad L., Dogterom M., Brunner D., Surrey T. 2007. Reconstitution of a microtubule plus-end tracking system in vitro.Nature. 450:1100–1105 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

