Steps and time to process clinical trials at the Cancer Therapy Evaluation Program
- PMID: 19255315
- PMCID: PMC2668703
- DOI: 10.1200/JCO.2008.19.9133
Steps and time to process clinical trials at the Cancer Therapy Evaluation Program
Abstract
Purpose: To examine the processes and document the calendar time required for the National Cancer Institute's Cancer Therapy Evaluation Program (CTEP) and Central Institutional Review Board (CIRB) to evaluate and approve phase III clinical trials.
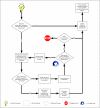
Methods: Process steps were documented by (1) interviewing CTEP and CIRB staff regarding the steps required to activate a trial from initial concept submission to trial activation by a cooperative group, (2) reviewing standard operating procedures, and (3) inspecting trial records and documents for selected trials to identify any additional steps. Calendar time was collected from initial concept submission to activation using retrospective data from the CTEP Protocol and Information Office.
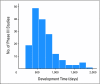
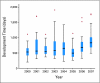
Results: At least 296 distinct processes are required for phase III trial activation: at least 239 working steps, 52 major decision points, 20 processing loops, and 11 stopping points. Of the 195 trials activated during the January 1, 2000, to December 31, 2007, study period, a sample of 167 (85.6%) was used for gathering timing data. Median calendar days from initial formal concept submission to CTEP to trial activation by a cooperative group was 602 days (interquartile range, 454 to 861 days). This time has not significantly changed over the past 8 years. There is a high variation in the time required to activate a clinical trial.
Conclusion: Because of their complexity, the overall development time for phase III clinical trials is lengthy, process laden, and highly variable. To streamline the process, a solution must be sought that includes all parties involved in developing trials.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
The ordinary miracle of cancer clinical trials.J Clin Oncol. 2009 Apr 10;27(11):1737-9. doi: 10.1200/JCO.2008.20.6292. Epub 2009 Mar 2. J Clin Oncol. 2009. PMID: 19255310 No abstract available.
References
-
- Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. - PubMed
-
- Comis RL, Miller JD, Aldige CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. - PubMed
-
- Dilts DM, Sandler AB. Invisible barriers to clinical trials: The impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24:4545–4552. - PubMed
-
- Dilts DM, Sandler AB, Baker M, et al. Processes to activate phase III clinical trials in a Cooperative Oncology Group: The Case of Cancer and Leukemia Group B. J Clin Oncol. 2006;24:4553–4557. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

