GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study
- PMID: 19255407
- PMCID: PMC2821836
- DOI: 10.1212/01.wnl.0000343880.13764.69
GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study
Abstract
Objective: To evaluate the safety and tolerability of natalizumab when added to glatiramer acetate (GA) in patients with relapsing multiple sclerosis. The primary outcome assessed whether this combination would increase the rate of development of new active lesions on cranial MRI scans vs GA alone.
Methods: This phase 2, randomized, double-blind, placebo-controlled study included patients aged 19 to 55 years who were treated with GA for at least 1 year before randomization and experienced at least one relapse during the previous year. Patients received IV natalizumab 300 mg (n = 55) or placebo (n = 55) once every 4 weeks plus GA 20 mg subcutaneously once daily for < or = 20 weeks.
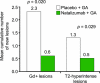
Results: The mean rate of development of new active lesions was 0.03 with combination therapy vs 0.11 with GA alone (p = 0.031). Combination therapy resulted in lower mean numbers of new gadolinium-enhancing lesions (0.6 vs 2.3 for GA alone, p = 0.020) and new/newly enlarging T2-hyperintense lesions (0.5 vs 1.3, p = 0.029). The incidence of infection and infusion reactions was similar in both groups; no hypersensitivity reactions were observed. One serious adverse event occurred with combination therapy (elective hip surgery). With the exception of an increase in anti-natalizumab antibodies with combination therapy, laboratory data were consistent with previous clinical studies of natalizumab alone.
Conclusion: The combination of natalizumab and glatiramer acetate seemed safe and well tolerated during 6 months of therapy.
Figures
References
-
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. - PubMed
-
- Johnson KP Brooks BR Cohen JA et al. for the Copolymer I Multiple Sclerosis Study Group. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology. 1995;45:1268–1276. - PubMed
-
- Jacobs LD Cookfair DL Rudick RA et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. - PubMed
-
- The Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis (PRISMS) Study Group. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. - PubMed
-
- Ffrench-Constant C Pathogenesis of multiple sclerosis. Lancet. 1994;343:271–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources