Epigenetic regulation of genetic integrity is reprogrammed during cloning
- PMID: 19255429
- PMCID: PMC2660773
- DOI: 10.1073/pnas.0900687106
Epigenetic regulation of genetic integrity is reprogrammed during cloning
Abstract
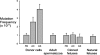
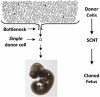
Cloning by somatic cell nuclear transfer (SCNT) circumvents processes that normally function during gametogenesis to prepare the gamete genomes to support development of new progeny following fertilization. One such process is enhanced maintenance of genetic integrity in germ cells, such that germ cells typically carry fewer spontaneously acquired mutations than somatic cells in the same individual. Thus, embryos produced from somatic cells by SCNT could directly inherit more mutations than naturally conceived embryos. Alternatively, they could inherit epigenetic programming that predisposes more rapid accumulation of de novo mutations during development. We used a transgenic mouse system to test these possibilities by producing cloned midgestation mouse fetuses from three different donor somatic cell types carrying significantly different initial frequencies of spontaneous mutations. We found that on an individual locus basis, mutations acquired spontaneously in a population of donor somatic cells are not likely to be propagated to cloned embryos by SCNT. In addition, we found that the rate of accumulation of spontaneous mutations was similar in fetuses produced by either natural conception or cloning, indicating that cloned fetuses do not acquire mutations more rapidly than naturally conceived fetuses. These results represent the first direct demonstration that the process of cloning by SCNT does not lead to an increase in the frequency of point mutations. These results also demonstrate that epigenetic mechanisms normally contribute to the regulation of genetic integrity in a tissue-specific manner, and that these mechanisms are subject to reprogramming during cloning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Maintenance of genetic integrity during natural and assisted reproduction.Reprod Biomed Online. 2009;18 Suppl 2:51-5. doi: 10.1016/s1472-6483(10)60449-x. Reprod Biomed Online. 2009. PMID: 19406032
-
Extranuclear Inheritance of Mitochondrial Genome and Epigenetic Reprogrammability of Chromosomal Telomeres in Somatic Cell Cloning of Mammals.Int J Mol Sci. 2021 Mar 18;22(6):3099. doi: 10.3390/ijms22063099. Int J Mol Sci. 2021. PMID: 33803567 Free PMC article. Review.
-
BIX-01294 increases pig cloning efficiency by improving epigenetic reprogramming of somatic cell nuclei.Reproduction. 2016 Jan;151(1):39-49. doi: 10.1530/REP-15-0460. Reproduction. 2016. PMID: 26604326
-
Locus-specific analysis of DNA methylation patterns in cloned and in vitro fertilized porcine embryos.J Reprod Dev. 2020 Dec 22;66(6):505-514. doi: 10.1262/jrd.2019-076. Epub 2020 Sep 8. J Reprod Dev. 2020. PMID: 32908081 Free PMC article.
-
H3.3 replacement facilitates epigenetic reprogramming of donor nuclei in somatic cell nuclear transfer embryos.Nucleus. 2014 Sep-Oct;5(5):369-75. doi: 10.4161/nucl.36231. Nucleus. 2014. PMID: 25482190 Review.
Cited by
-
Tertiary Epimutations - A Novel Aspect of Epigenetic Transgenerational Inheritance Promoting Genome Instability.PLoS One. 2016 Dec 19;11(12):e0168038. doi: 10.1371/journal.pone.0168038. eCollection 2016. PLoS One. 2016. PMID: 27992467 Free PMC article.
-
Enhanced genetic integrity in mouse germ cells.Biol Reprod. 2013 Jan 3;88(1):6. doi: 10.1095/biolreprod.112.103481. Print 2013 Jan. Biol Reprod. 2013. PMID: 23153565 Free PMC article.
-
Dynamic Variations in Genetic Integrity Accompany Changes in Cell Fate.Stem Cells Dev. 2016 Nov 15;25(22):1698-1708. doi: 10.1089/scd.2016.0221. Epub 2016 Oct 12. Stem Cells Dev. 2016. PMID: 27627671 Free PMC article.
-
Regenerant Arabidopsis lineages display a distinct genome-wide spectrum of mutations conferring variant phenotypes.Curr Biol. 2011 Aug 23;21(16):1385-90. doi: 10.1016/j.cub.2011.07.002. Epub 2011 Jul 28. Curr Biol. 2011. PMID: 21802297 Free PMC article.
References
-
- Jaenisch R. Human cloning–the science and ethics of nuclear transplantation. N Engl J Med. 2004;351:2787–2791. - PubMed
-
- Meissner A, Jaenisch R. Mammalian nuclear transfer. Dev Dyn. 2006;235:2460–2469. - PubMed
-
- Westhusin ME, Shin T, Templeton JW, Burghardt RC, Adams LG. Rescuing valuable genomes by animal cloning: a case for natural disease resistance in cattle. J Anim Sci. 2007;85:138–142. - PubMed
-
- Williams JB, Shin T, Liu L, Flores-Foxworth G, Romano J, Blue-McClendon A, Kraemer D, Westhusin ME. Cloning of exotic/endangered species: desert bighorm sheep. Methods Mol Biol. 2006;348:169–182. - PubMed
-
- Westhusin M, Hinrichs K, Choi YH, Shin T, Liu L, Kraemer D. Cloning companion animals (horses, cats, and dogs) J Anim Sci. 2003;5:301–317. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

