Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis
- PMID: 19255431
- PMCID: PMC2657396
- DOI: 10.1073/pnas.0810067106
Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis
Abstract
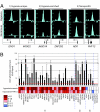
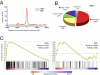
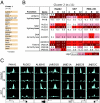
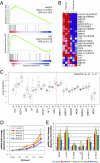
Adaptation to hypoxia is mediated through a coordinated transcriptional response driven largely by hypoxia-inducible factor 1 (HIF-1). We used ChIP-chip and gene expression profiling to identify direct targets of HIF-1 transactivation on a genome-wide scale. Several hundred direct HIF-1 targets were identified and, as expected, were highly enriched for proteins that facilitate metabolic adaptation to hypoxia. Surprisingly, there was also striking enrichment for the family of 2-oxoglutarate dioxygenases, including the jumonji-domain histone demethylases. We demonstrate that these histone demethylases are direct HIF targets, and their up-regulation helps maintain epigenetic homeostasis under hypoxic conditions. These results suggest that the coordinated increase in expression of several oxygen-dependent enzymes by HIF may help compensate for decreased levels of oxygen under conditions of cellular hypoxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. - PubMed
-
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. - PubMed
-
- Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. - PubMed
-
- Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

