A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids
- PMID: 19255658
- PMCID: PMC2706488
- DOI: 10.1039/b814322f
A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids
Abstract
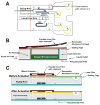
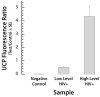
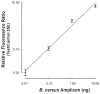
An inexpensive, hand-held, point-of-care, disposable, self-contained immunoassay cassette comprised of air pouches for pumping, a metering chamber, reagents storage chambers, a mixer, and a lateral flow strip was designed, constructed, and tested. The assay was carried out in a consecutive flow format. The detection was facilitated with up-converting phosphor (UCP) reporter particles. The automated, timely pumping of the various reagents was driven by a spring-loaded timer. The utility of the cassette was demonstrated by detecting antibodies to HIV in saliva samples and further evaluated with a non-contagious, haptenized DNA assay. The cassette has several advantages over dip sticks such as sample preprocessing, integrated storage of reagents, and automated operation that reduces operator errors and training. The cassette and actuator described herein can readily be extended to detect biomarkers of other diseases in body fluids and other fluids at the point of care. The system is particularly suitable for resource-poor countries, where funds and trained personnel are in short supply.
Figures







References
-
- Wang J, Chen Z, Corstjens PLAM, Mauk MG, Bau HH. Lab Chip. 2006;6:46–53. - PubMed
-
- Srinivasan V, Pamula VK, Fair RB. Lab Chip. 2004;4:310–315. - PubMed
-
- Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412–418. - PubMed
-
- Chen X, Cui D, Liu C. Electrophoresis. 2008;29:1844–1851. - PubMed
-
- Garcia E, Kirkham JR, Hatch AV, Hawkins KR, Yager P. Lab Chip. 2004;4:78–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

