The cardiac sodium channel displays differential distribution in the conduction system and transmural heterogeneity in the murine ventricular myocardium
- PMID: 19255801
- PMCID: PMC2722719
- DOI: 10.1007/s00395-009-0012-8
The cardiac sodium channel displays differential distribution in the conduction system and transmural heterogeneity in the murine ventricular myocardium
Abstract
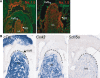
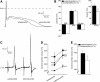
Cardiac sodium channels are responsible for conduction in the normal and diseased heart. We aimed to investigate regional and transmural distribution of sodium channel expression and function in the myocardium. Sodium channel Scn5a mRNA and Na(v)1.5 protein distribution was investigated in adult and embryonic mouse heart through immunohistochemistry and in situ hybridization. Functional sodium channel availability in subepicardial and subendocardial myocytes was assessed using patch-clamp technique. Adult and embryonic (ED14.5) mouse heart sections showed low expression of Na(v)1.5 in the HCN4-positive sinoatrial and atrioventricular nodes. In contrast, high expression levels of Na(v)1.5 were observed in the HCN4-positive and Cx43-negative AV or His bundle, bundle branches and Purkinje fibers. In both ventricles, a transmural gradient was observed, with a low Na(v)1.5 labeling intensity in the subepicardium as compared to the subendocardium. Similar Scn5a mRNA expression patterns were observed on in situ hybridization of embryonic and adult tissue. Maximal action potential upstroke velocity was significantly lower in subepicardial myocytes (mean +/- SEM 309 +/- 32 V/s; n = 14) compared to subendocardial myocytes (394 +/- 32 V/s; n = 11; P < 0.05), indicating decreased sodium channel availability in subepicardium compared to subendocardium. Scn5a and Na(v)1.5 show heterogeneous distribution patterns within the cardiac conduction system and across the ventricular wall. This differential distribution of the cardiac sodium channel may have profound consequences for conduction disease phenotypes and arrhythmogenesis in the setting of sodium channel disease.
Figures




References
-
- Bébarová M, O’Hara T, Geelen JL, Jongbloed RJ, Timmermans C, Arens YH, Rodriguez LM, Rudy Y, Volders PG (2008) Subepicardial phase 0 block and discontinuous transmural conduction underlie right precordial ST-segment elevation by a SCN5A loss-of-function mutation. Am J Physiol Heart Circ Physiol 295:H48–H58 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

