Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells
- PMID: 19255807
- PMCID: PMC2674203
- DOI: 10.1007/s10162-009-0160-4
Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells
Abstract
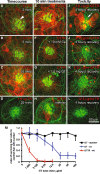
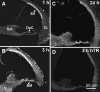
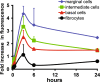
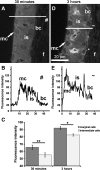
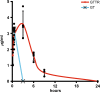
Aminoglycosides enter inner ear hair cells across their apical membranes via endocytosis, or through the mechanoelectrical transduction channels in vitro, suggesting that these drugs enter cochlear hair cells from endolymph to exert their cytotoxic effect. We used zebrafish to determine if fluorescently tagged gentamicin (GTTR) also enters hair cells via apically located calcium-sensitive cation channels and the cytotoxicity of GTTR to hair cells. We then examined the serum kinetics of GTTR following systemic injection in mice and which murine cochlear sites preferentially loaded with systemically administered GTTR over time by confocal microscopy. GTTR is taken up by, and is toxic to, wild-type zebrafish neuromast hair cells. Neuromast hair cell uptake of GTTR is attenuated by high concentrations of extracellular calcium or unconjugated gentamicin and is blocked in mariner mutant zebrafish, suggestive of entry via the apical mechanotransduction channel. In murine cochleae, GTTR is preferentially taken up by the stria vascularis compared to the spiral ligament, peaking 3 h after intra-peritoneal injection, following GTTR kinetics in serum. Strial marginal cells display greater intensity of GTTR fluorescence compared to intermediate and basal cells. Immunofluorescent detection of gentamicin in the cochlea also revealed widespread cellular labeling throughout the cochlea, with preferential labeling of marginal cells. Only GTTR fluorescence displayed increasing cytoplasmic intensity with increasing concentration, unlike the cytoplasmic intensity of fluorescence from immunolabeled gentamicin. These data suggest that systemically administered aminoglycosides are trafficked from strial capillaries into marginal cells and clear into endolymph. If so, this will facilitate electrophoretically driven aminoglycoside entry into hair cells from endolymph. Trans-strial trafficking of aminoglycosides from strial capillaries to marginal cells will be dependent on as-yet-unidentified mechanisms that convey these drugs across the intra-strial electrical barrier and into marginal cells.
Figures





Similar articles
-
Competitive antagonism of fluorescent gentamicin uptake in the cochlea.Hear Res. 2010 Sep 1;268(1-2):250-9. doi: 10.1016/j.heares.2010.06.008. Epub 2010 Jun 16. Hear Res. 2010. PMID: 20561573 Free PMC article.
-
A systemic gentamicin pathway across the stria vascularis.Hear Res. 2008 Jan;235(1-2):114-24. doi: 10.1016/j.heares.2007.10.010. Epub 2007 Nov 17. Hear Res. 2008. PMID: 18082985 Free PMC article.
-
Systemic Fluorescent Gentamicin Enters Neonatal Mouse Hair Cells Predominantly Through Sensory Mechanoelectrical Transduction Channels.J Assoc Res Otolaryngol. 2020 Apr;21(2):137-149. doi: 10.1007/s10162-020-00746-3. Epub 2020 Mar 9. J Assoc Res Otolaryngol. 2020. PMID: 32152768 Free PMC article.
-
Principles in cochlear toxicity.Arch Toxicol Suppl. 1985;8:221-39. doi: 10.1007/978-3-642-69928-3_35. Arch Toxicol Suppl. 1985. PMID: 3913402 Review.
-
Adrenergic and muscarinic control of cochlear endolymph production.Adv Otorhinolaryngol. 2002;59:42-50. doi: 10.1159/000059255. Adv Otorhinolaryngol. 2002. PMID: 11885660 Review.
Cited by
-
Localization of gentamicin uptake in the acutely isolated inner ear of the rat.Int J Physiol Pathophysiol Pharmacol. 2011;3(2):71-87. Epub 2011 Mar 28. Int J Physiol Pathophysiol Pharmacol. 2011. PMID: 21760966 Free PMC article.
-
Aminoglycoside- and glycopeptide-induced ototoxicity in children: a systematic review.JAC Antimicrob Resist. 2021 Dec 14;3(4):dlab184. doi: 10.1093/jacamr/dlab184. eCollection 2021 Dec. JAC Antimicrob Resist. 2021. PMID: 34917943 Free PMC article. Review.
-
Functional and structural changes throughout the auditory system following congenital and early-onset deafness: implications for hearing restoration.Front Syst Neurosci. 2013 Nov 26;7:92. doi: 10.3389/fnsys.2013.00092. Front Syst Neurosci. 2013. PMID: 24324409 Free PMC article. Review.
-
TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells.Neuron. 2018 Aug 22;99(4):736-753.e6. doi: 10.1016/j.neuron.2018.07.033. Neuron. 2018. PMID: 30138589 Free PMC article.
-
Intratympanic Lipopolysaccharide Elevates Systemic Fluorescent Gentamicin Uptake in the Cochlea.Laryngoscope. 2021 Sep;131(9):E2573-E2582. doi: 10.1002/lary.29610. Epub 2021 May 6. Laryngoscope. 2021. PMID: 33956344 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1749-6632.1999.tb08636.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1749-6632.1999.tb08636.x'}, {'type': 'PubMed', 'value': '10842584', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10842584/'}]}
- Aran JM, Erre JP, Lima da Costa D, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Ann N Y Acad Sci 884:60–8, 1999. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '4193456', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/4193456/'}]}
- Balogh K, Jr, Hiraide F, Ishii D. Distribution of radioactive dihydrostreptomycin in the cochlea. An autoradiographic study. Ann. Otol. Rhinol. Laryngol 79:641–52, 1970. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF00495788', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf00495788'}, {'type': 'PubMed', 'value': '3011710', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/3011710/'}]}
- Bareggi R, Narducci P, Grill V, Mallardi F, Zweyer M, Fusaroli P. Localization of an aminoglycoside (streptomycin) in the inner ear after its systemic administration. A histochemical study using fluorescence microscopy. Histochemistry 84:237–40, 1986. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '659353', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/659353/'}]}
- Brummett RE, Fox KE, Bendrick TW, Himes DL. Ototoxicity of tobramycin, gentamicin, amikacin and sisomicin in the guinea pig. J. Antimicrob. Chemother 4(Suppl A):73–83, 1978. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16805980', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16805980/'}]}
- Chu HQ, Xiong H, Zhou XQ, Han F, Wu ZG, Zhang P, Huang XW, Cui YH. Aminoglycoside ototoxicity in three murine strains and effects on NKCC1 of stria vascularis. Chin. Med. J. (Engl) 119:980–5, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

