Toll-like receptors in skin
- PMID: 19256306
- PMCID: PMC2633625
- DOI: 10.1016/j.yadr.2008.09.004
Toll-like receptors in skin
Abstract
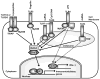
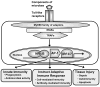
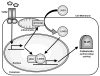
TLRs have emerged as a major class of PRRs that are involved in detecting invading pathogens in the skin and initiating cutaneous immune responses. TLRs are expressed on many different cell types in the skin, including keratinocytes and Langerhans cells in the epidermis. Each TLR can recognize a different microbial component and there are differences among the TLR signaling pathways, which lead to distinct immune responses against a given pathogen. Certain TLRs have been implicated in the pathogenesis of skin diseases, such as atopic dermatitis, psoriasis, and acne vulgaris. In addition, TLRs have been shown to be important in cutaneous host defense mechanisms against common bacterial, fungal, and viral pathogens in the skin, such as S aureus, C albicans, and HSV. Since the discovery that topical TLR agonists promote antiviral and antitumor immune responses, there has been considerable interest in the development of TLR-based therapies for skin diseases, skin cancer, and infections. Future research involving TLRs in skin will hopefully provide new insights into host defense against skin pathogens and novel therapeutic targets aimed at treating skin disease and skin cancer.
Figures

References
-
- Kang SS, Kauls LS, Gaspari AA. Toll-like receptors: applications to dermatologic disease. J Am Acad Dermatol. 2006;54(6):951–983. - PubMed
-
- McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125(1):1–8. - PubMed
-
- Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29(1):15–26. - PubMed
-
- Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125(4):629–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
