Catalytic properties of botulinum neurotoxin subtypes A3 and A4
- PMID: 19256469
- PMCID: PMC2701208
- DOI: 10.1021/bi801686b
Catalytic properties of botulinum neurotoxin subtypes A3 and A4
Abstract
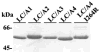
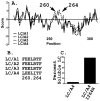
Botulinum toxins (BoNT) are zinc proteases (serotypes A-G) which cause flaccid paralysis through the cleavage of SNARE proteins within motor neurons. BoNT/A was originally organized into two subtypes, BoNT/A1 and BoNT/A2, which are approximately 95% homologous and possess similar catalytic activities. Subsequently, two additional subtypes were identified, BoNT/A3 (Loch Maree) and BoNT/A4 (657Ba), which are 81 and 88% homologous with BoNT/A1, respectively. Alignment studies predicted that BoNT/A3 and BoNT/A4 were sufficiently different from BoNT/A1 to affect SNAP25 binding and cleavage. Recombinant light chain (LC) of BoNT/A3 (LC/A3) and BoNT/A4 (LC/A4) were subjected to biochemical analysis. LC/A3 cleaved SNAP25 at 50% of the rate of LC/A1 but cleaved SNAPtide at a faster rate than LC/A1, while LC/A4 cleaved SNAP25 and SNAPtide at slower rates than LC/A1. LC/A3 and LC/A4 had similar K(m) values for SNAP25 relative to LC/A1, while the k(cat) for LC/A4 was 10-fold slower than that for LC/A1, suggesting a defect in substrate cleavage. Neither LC/A3 nor LC/A4 possessed autocatalytic activity, a property of LC/A1 and LC/A2. Thus, the four subtypes of BoNT/A bind SNAP25 with similar affinity but have different catalytic capacities for SNAP25 cleavage, SNAPtide cleavage, and autocatalysis. The catalytic properties identified among the subtypes of LC/A may influence strategies for the development of small molecule or peptide inhibitors as therapies against botulism.
Figures
References
-
- Shiva G, Rossetti O, Santucci A, DasGupta RR, Montecucco C. Botulinum Neurotoxins are Zinc Proteases. Journal of Biological Chemistry. 1992;267:23479–23483. - PubMed
-
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem. 1994;269:1617–1620. - PubMed
-
- Naumann M, So Y, Argoff CE, Childers MK, Dykstra DD, Gronseth GS, Jabbari B, Kaufmann HC, Schurch B, Silberstein SD, Simpson DM. Assessment: Botulinum neurotoxin in the treatment of autonomic disorders and pain (an evidence-based review): Reports of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1707–1714. - PubMed
-
- Bandyopadhyay S, Clark AW, DasGupta BR, Sathyamoorthy V. Role of the heavy and light chains of botulinum neurotoxin in neuromuscular paralysis. J Biol Chem. 1989;262:2660–2663. - PubMed
-
- Lacy DB, Stevens RC. Sequence homology and structural analysis of the clostridial neurotoxins. J Mol Biol. 1999;291:1091–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

